Saving Lives with Data
Regulatory grade safety and effectiveness data replacing trials and registries
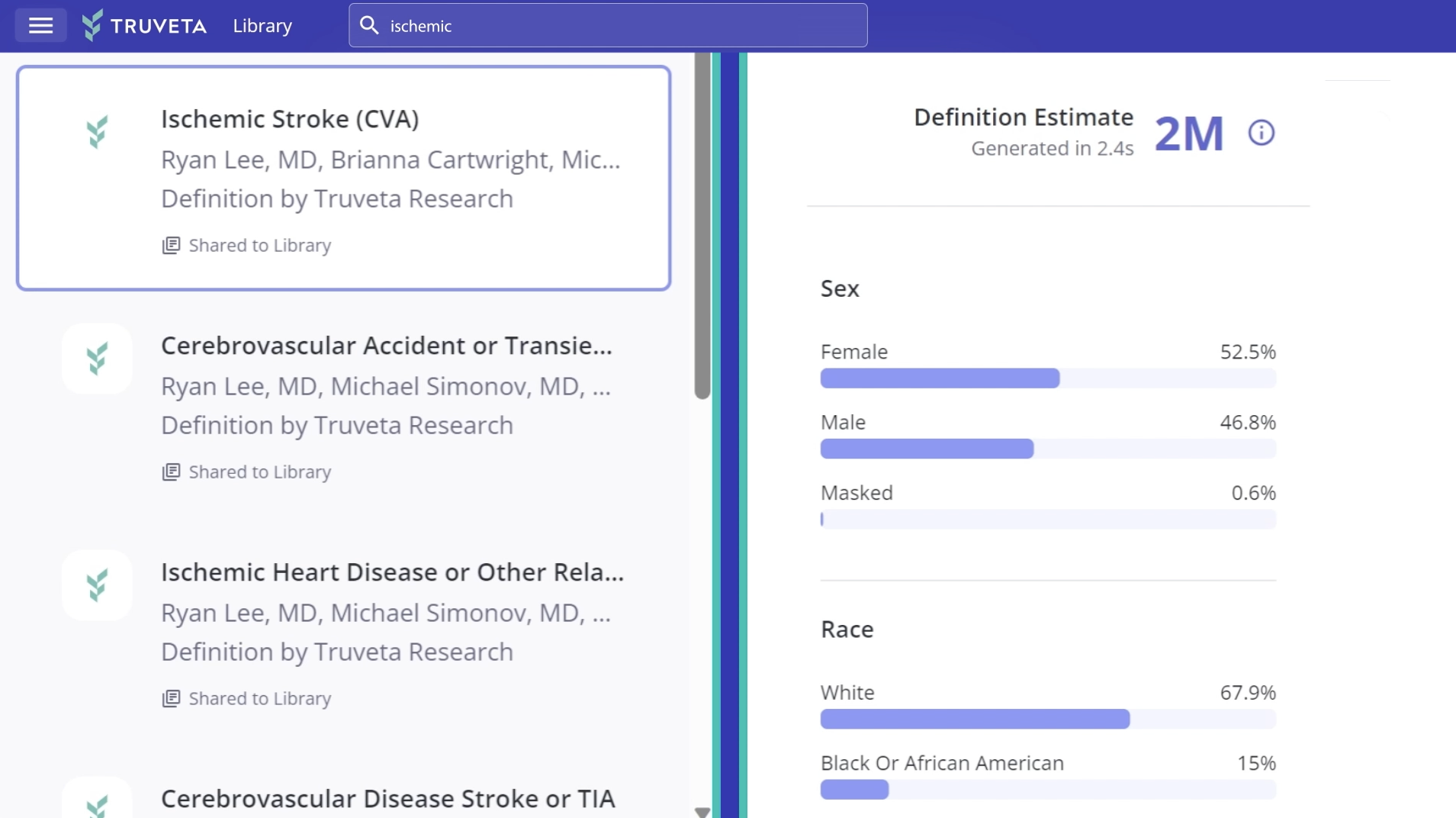
Accelerate therapy adoption with the most representative, complete, and timely patient journey data
EHR data from more than 130 million patients, including clinical notes and images, linked with closed claims of 200 million patients.
Trusted by leading life science, public health, and healthcare organizations

“Truveta enables us to gather insights on a breadth of devices and disease states and further our commitment to addressing the disparities in access to healthcare that exist across various patient populations and demographics.”
Michael R. Jaff, DO
Chief Medical Officer and Vice President, Medical Affairs, Innovation and Technology, Peripheral Interventions, Boston Scientific
Accelerate therapy adoption and advance patient care

Safety

HEOR

Clinical trials

R&D

Close care gaps
Leverage real-world data, powerful analytics, and the Truveta Language model to support therapy development and access, care quality improvement, and public health.
Leverage real-world data, powerful analytics, and the Truveta Language model to support therapy development and access, care quality improvement, and public health.

Safety

HEOR

Clinical trials

R&D

Close care gaps


“The world needs Truveta because information is key to solving healthcare problems. With Truveta, we have a national de-identified dataset, representative of the diversity of our country, from which we can learn faster to improve care in our communities.”
Rod Hochman, MD
CEO Emeritus, Providence
Chair, Truveta Board of Directors
Truveta is a growing collective of 30 health systems with a shared mission of Saving Lives with Data



























































Featured in