Real-world evidence solutions
Truveta’s patient-level health data empowers researchers to generate evidence to drive product innovation and clinical advancements
How we can help
Clinical trials
Clinical trials are costly, and only 9.6% of drugs and 45% of devices are approved. Truveta enables life sciences companies to:
- De-risk clinical programs by optimizing trial design
- Recruit faster and more equitably with Truveta’s health system network
- Generate robust evidence using real-world control arms
Testing I/E criteria to de-risk clinical programs

Sample heart failure population with inclusion/exclusion criteria applied
Safety monitoring
Post-approval safety studies are costly, as are the consequences of delayed signal detection. Truveta enables life sciences companies to:
- Replace expensive and long-running registries
- Meet regulatory evidence standards faster and at lower cost
- Assess the validity of potential safety signals in real-time
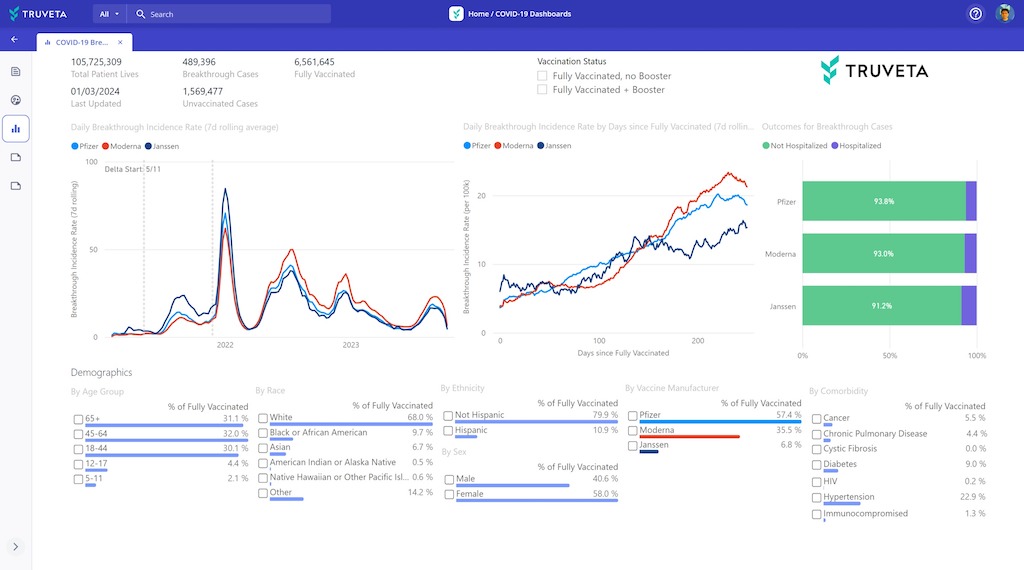
Tracking adverse events post-vaccination

Dashboard showing adverse events by demographic, manufacturer, comorbidity, dose, and more within Truveta Studio
Comparative effectiveness
The absence of real-world outcomes data may result in reduced clinician confidence and product adoption. Truveta enables life sciences to:
- Generate evidence of differentiated clinical outcomes
- Understand real-world product performance for specific subpopulations
- Ensure the right patients have access to therapies
Comparing outcomes of novel interventions

Comparative bleeding incidence 7 days post-procedure for pulmonary embolism interventions
Label expansion
Missed label expansion opportunities may result in unmet patient needs and limit R&D ROI. Truveta enables life sciences companies to:
- Understand real-world product use with full clinical context
- Produce regulatory-grade evidence for label expansion
- Replace clinical studies with long lead times
Monitoring on- and off-label prescribing patterns

First-time GLP-1 prescriptions by medication by labeled indication and on- and off-label use
Closing care gaps
Suboptimal treatment patterns and ineffective care coordination drive poorer health outcomes and significant avoidable costs. Truveta’s unique relationship with community health systems enables life sciences companies to:
- Accelerate discoveries by streamlining evidence generation
- Partner on novel data collection to understand current clinical context
- Collaborate on groundbreaking research to improve patient outcomes
Assessing the clinical consequences of diagnostic delays

Rates of mental health diagnoses and surgery by length of HS diagnostic delay
How we can help

Clinical trials

Safety monitoring

Comparative effectiveness

Label expansion

Closing care gaps
Clinical trials
Accelerate clinical trial timelines and speed time to market by identifying the right patients faster.

Safety monitoring
Fulfill post-market safety requirements at half the cost and twice as fast with regulatory-grade data.
Comparative effectiveness
Establish product differentiation and increase market adoption with timely patient-level outcomes and SDOH data.

Label expansion
Identify emerging opportunities to expand product usage and improve care with timely patient-level outcomes data.

Closing care gaps
Collaborate with health systems to advance adoption of insights into patient care.

Learn why top pharmaceutical and med device companies use Truveta to:
- Drive better healthcare strategies with the most complete picture of US health
- Make decisions ahead of the competition with data that's refreshed daily
- Unlock meaningful insights from clinical notes and medical images
- Boost collaboration with seamless data analytics for the entire team
- Let’s work together to improve patient outcomes with data.
