US spending on medical devices and in-vitro diagnostics totals more than $199 billion a year, with most of the costs associated with clinical development. Label expansion provides a pathway for recouping costs associated with the device development process by increasing product utilization, extending patent life, and diversifying revenue streams. This is especially true when real-world data is used to support label expansion, rather than clinical trial data – as this information can be obtained more quickly and cost effectively.
In part two of this label expansion series (see part 1: off-label use in medical practice), we explore frequently asked questions related to real-world data and label expansion for medical device companies.
What are the benefits of real-world data for device companies?
When comparing clinical trials versus real-world data as a source of evidence on product safety, use, and effectiveness, real-world data offers several key advantages. Namely, real-world data can enable researchers to:
- Evaluate a broader range of clinical questions
- Include populations otherwise underrepresented in clinical studies
- Assess longer-term outcomes associated with device use, by allowing for studies of a longer duration
- Overcome issues of impractical, challenging, or unethical study design (e.g., issues around treatment assignment) by assessing naturally occurring, real-world device use
- Incorporate patient experience data, which may provide new insight into device performance
- Inform future device modifications and new technology development by better aligning with device innovation cycles
- Inform device risk-benefit profile assessment for real-world populations
In fact, the FDA’s draft guidance on using real-world evidence to support regulatory decision-making for medical devices, issued in December 2023, notes that “analyses of real-world data, using appropriate methods, may in some cases provide similar information with comparable or even superior characteristics to information collected and analyzed through a traditional clinical study.”
What real-world data sources can device companies use for evidence generation, and what are the pros and cons?
Medical device companies have a range of real-world data sources at their disposal, each with unique advantages and drawbacks. The primary real-world data sources used by device companies are claims, registries, and EHR data.
Claims data provide detailed billing and payment information and large sample sizes, but lack detail on clinical outcomes, patient journeys, and the surrounding clinical context for devices. Additionally, claims datasets typically have time lags of 6-12 months and may be subject to bias as they are optimized for reimbursement.
Registries collect standardized data on specialized patient populations with long-term follow-up but are often highly resource-intensive and subject to selection bias. For registry data collected prospectively, the results may not be available for years.
Electronic health records (EHRs) offer comprehensive patient records and rich clinical information, but these data sources have historically been challenging to work with since they are typically fragmented, inaccessible, and unstructured. Additionally, device data capture is not standardized in clinical practice, so EHR documentation typically lacks brand-, device-, and version-specific identifiers. This prevents researchers from studying individual devices at scale and instead limits them to studying broad device classes or setting up agreements with individual providers or health systems to collect necessary data. Advancements in AI have changed this, however – unlocking access to the full scope of data contained in EHRs, including clinical notes, images, and unique device identifiers.
How does Truveta help with label expansion?
- Understanding real-world product use with full clinical context
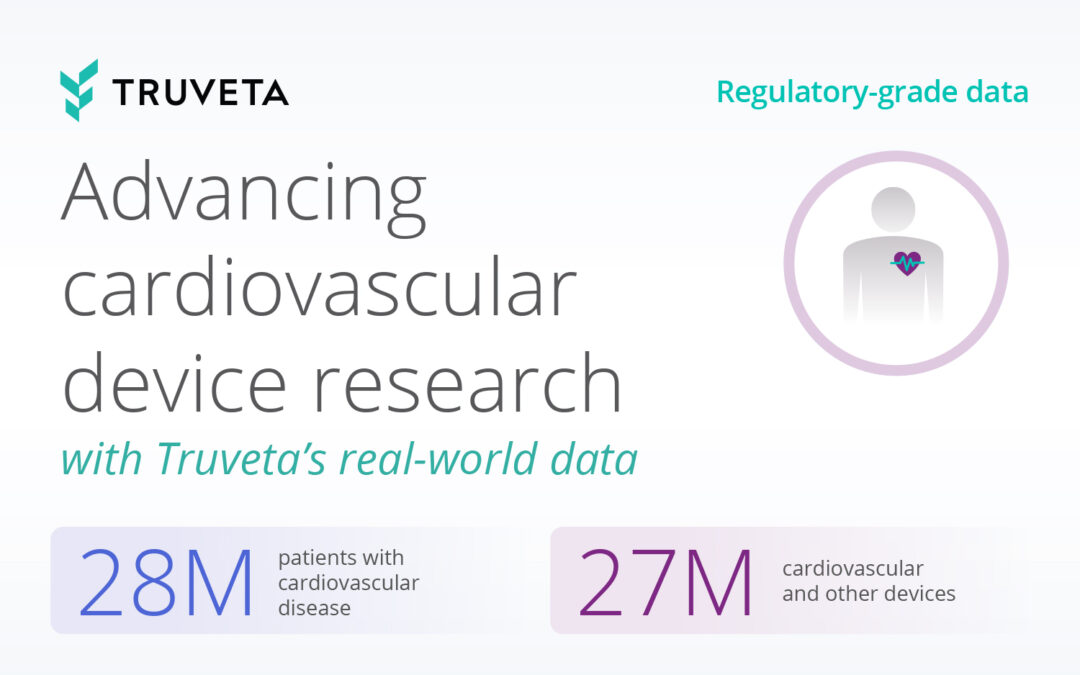
Truveta provides regulatory-grade EHR data for more than 100 million patients, linked with claims, social drivers of health (SDOH), and mortality data for a complete view into the patient journey. This is possible because of the Truveta Language Model (TLM), a large-language, multi-modal AI model trained on complete medical records and used to clean and standardize billions of daily healthcare data points for research.
In the case of devices, TLM standardizes device information to the Truveta device hierarchy, which includes the company and brand specific information necessary for research that standard terminologies often lack. The device hierarchy is based on three fields provided in the GUDID: company name, brand name, and product code name (associated with the FDA product code assigned to a specific device); with the UDIs associated where available. Truveta Data currently includes more than 3.5 million device keys and more than 70 million records mapped to more than 188,000 unique device identifiers.
Access to device-level data in the context of the full medical record – which includes clinical notes, images, lab results, and procedure logs for a nationally representative population – enables researchers can better understand when, how, and by whom devices are used in the real-world, including for off-label uses.
- Producing regulatory-grade evidence for label expansion
With visibility into the patients using specific devices and their associated clinical outcomes, manufacturers can identify potential candidate populations for label expansion. Procedure logs, imaging data, and clinical notes may also provide insight into off-label device insertion or delivery methods that may warrant label expansion consideration.
The FDA is increasingly open to considering real-world data as part of label expansion submissions, especially if the data demonstrate meaningful clinical benefits and safety profiles. Researchers using Truveta Studio will soon be able to denote which studies conducted on the platform will be submitted to regulators, enabling them to easily and automatically compile all the regulatory documents needed for submission – eliminating manual processes.
- Replacing clinical studies with long lead times
In its December 2023 draft guidance, the FDA notes that “under the right conditions, real-world evidence may be suitable to support the marketing authorization of a new device or the expansion of the indications for use of devices already on the market.”
Compared to traditional clinical studies, leveraging real-world data for label expansion can potentially lead to shorter lead times for regulatory approval. Since the data reflects real-world usage and outcomes, regulators may be more inclined to expedite the approval process, especially if the evidence is robust and compelling.
Truveta Data is complete, representative, and timely, enabling scientifically rigorous research across all diseases, drugs, and devices. Learn more about the robust data quality processes, analytics capabilities, data security, and patient privacy policies underlying our offering.
Accelerating medical device product adoption with real-world data
Incorporating real-world data into label expansion efforts empowers medical device companies to deliver innovative solutions that address evolving healthcare challenges and improve patient outcomes. Contact us to learn more or request a demo.