Authors: Brianna M. Goodwin Cartwright, MS ⊕Truveta, Inc, Bellevue, WA, Patricia J. Rodriguez, PhD MPH ⊕Truveta, Inc, Bellevue, WA, Samuel Gratzl, PhD ⊕Truveta, Inc, Bellevue, WA, Charlotte Baker, DrPH MPH CPH ⊕Truveta, Inc, Bellevue, WA, Nicholas Stucky, MD PhD ⊕Truveta, Inc, Bellevue, WA

- Little is known about the uptake of lecanemab and the associated demographics and social drivers of health (SDOH) of patients who are prescribed and/or receiving the medication approved for treating Alzheimer’s disease.
- While the number of patients receiving lecanemab have increased since the medication received FDA approval in July 2023, uptake remains limited.
- There are significant differences in the age group, race, income, and marital status between patients with lecanemab prescriptions and patients with first-time Alzheimer’s diagnoses during the study period.
This blog is an extension of our poster presented at ISPOR 2024, titled Real-world prescribing of lecanemab in the United States.
Lecanemab (brand name Leqembi) received accelerated FDA approval in January 2023 (US Food & Drug Administration, 2023a) and full FDA approval in July 2023 (US Food & Drug Administration, 2023b) for the treatment of Alzheimer’s disease. After full approval, CMS (Medicare) approved reimbursement for lecanemab, and patients have started to receive the drug as a part of their care. However, lecanemab is extremely expensive. With an annual list price of $26,500 (Alzheimer’s Association, 2024; Institute for Clinical and Economic Review, 2023), the price exceeds the estimated range for cost effectiveness (Institute for Clinical and Economic Review, 2023).
We’ve previously described trends in APOE genetic testing associated with lecanemab and an initial population with lecanemab prescriptions with data through October 2023.
Little is known about the continued uptake of lecanemab and the associated demographics and social drivers of health (SDOH) of patients who are prescribed this medication. With the high price point and increased availability of the drug since our last analysis, we explored the uptake, demographics, and SDOH factors of US adults prescribed lecanemab, compared to the general adult population diagnosed with Alzheimer’s disease during the study period.
You can also view this study directly in Truveta Studio.
Methods
Using a subset of Truveta, we defined two populations:
- Lecanemab population: Adults with a lecanemab prescription (received a prescription, administration, or claim) between January 1, 2023 and February 29, 2024
- Population with Alzheimer’s disease: Adults with a first-time diagnosis of Alzheimer’s disease between January 1, 2023 and February 29, 2024
Patients under age 18 were excluded from both populations. The populations were defined separately but may overlap.
We describe the uptake patterns of lecanemab over time. We also describe the differences in demographics and SDOH between the lecanemab population and population with Alzheimer’s disease. We studied SDOH variables of individual income, marital status, if a person rented or owned their place of living, and housing stability. Stable housing was defined as one or fewer moves within the previous year; unstable housing was defined as two or more moves within the previous year. Chi squared tests were used to compare differences between the populations.
Results
We found 408 adults with lecanemab prescriptions and 63,002 adults with a first-time Alzheimer’s diagnosis within the study period.
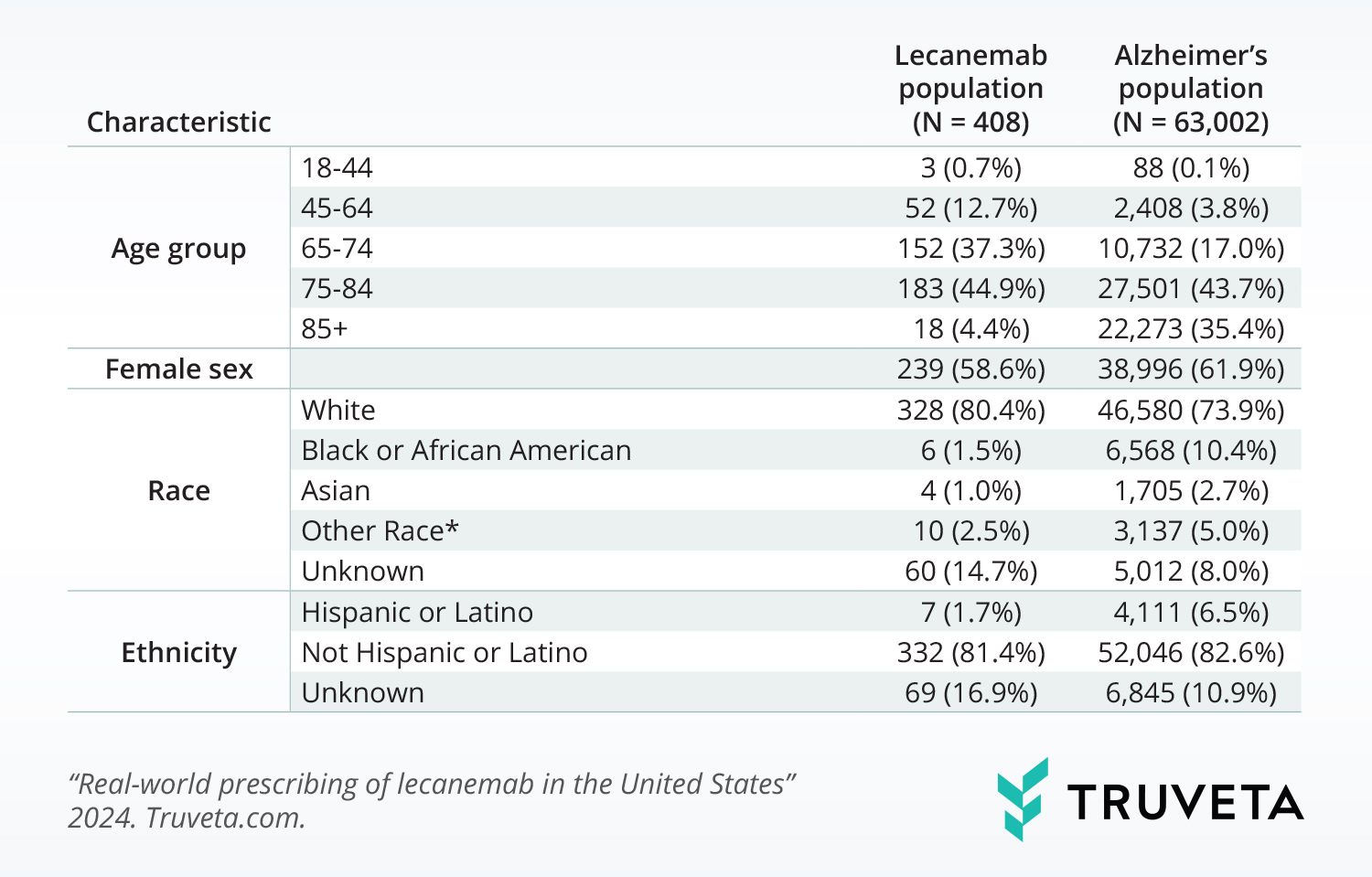
Demographic distributions
The population with a lecanemab prescription had a significantly different demographic makeup than the population with Alzheimer’s. The populations differed in the following ways:
- Age group (p< 0.001): The lecanemab population was made up of a larger percentage of people under 74 years of age.
- Race (p<0.001): The lecanemab population was made up of a larger percentage of white individuals and a smaller percentage of Black or African American individuals.
- Ethnicity (p<0.001): The lecanemab population was made up of a smaller percentage of people with Hispanic or Latino ethnicity.
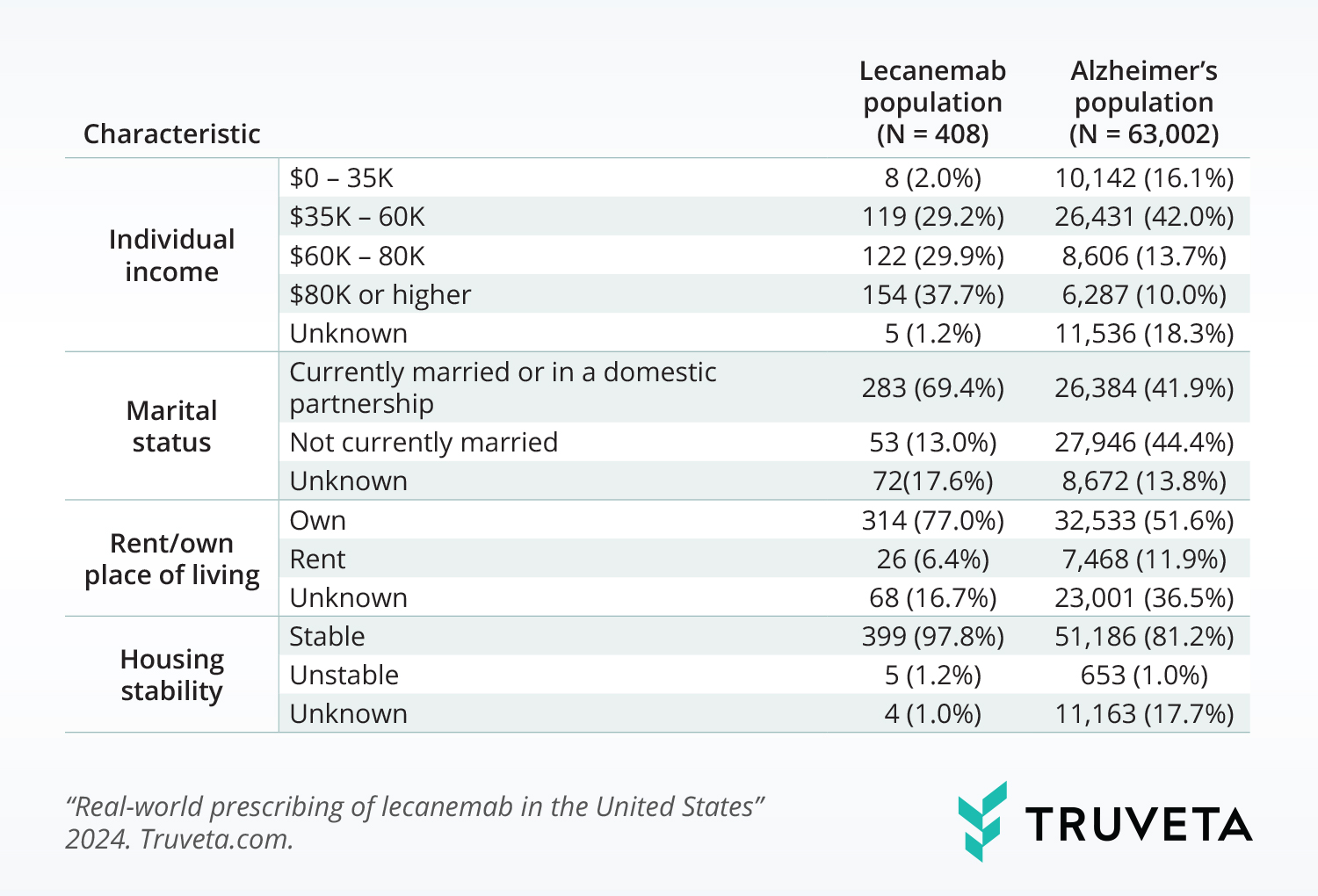
SDOH distributions
The population with lecanemab prescriptions also differed from the population with Alzheimer’s in their SDOH distributions. The populations differed in the following ways:
- Individual income (p<0.001): A larger percentage of the lecanemab population had incomes over $80K.
- Marital status (p <0.001): A larger percentage of the lecanemab population was married or in a partnership.
- Rent/own place of living (p<0.001): A larger percentage of the lecanemab population owned their place of living.
- Housing stability (p <0.001): A larger percentage of the lecanemab population had stable housing.
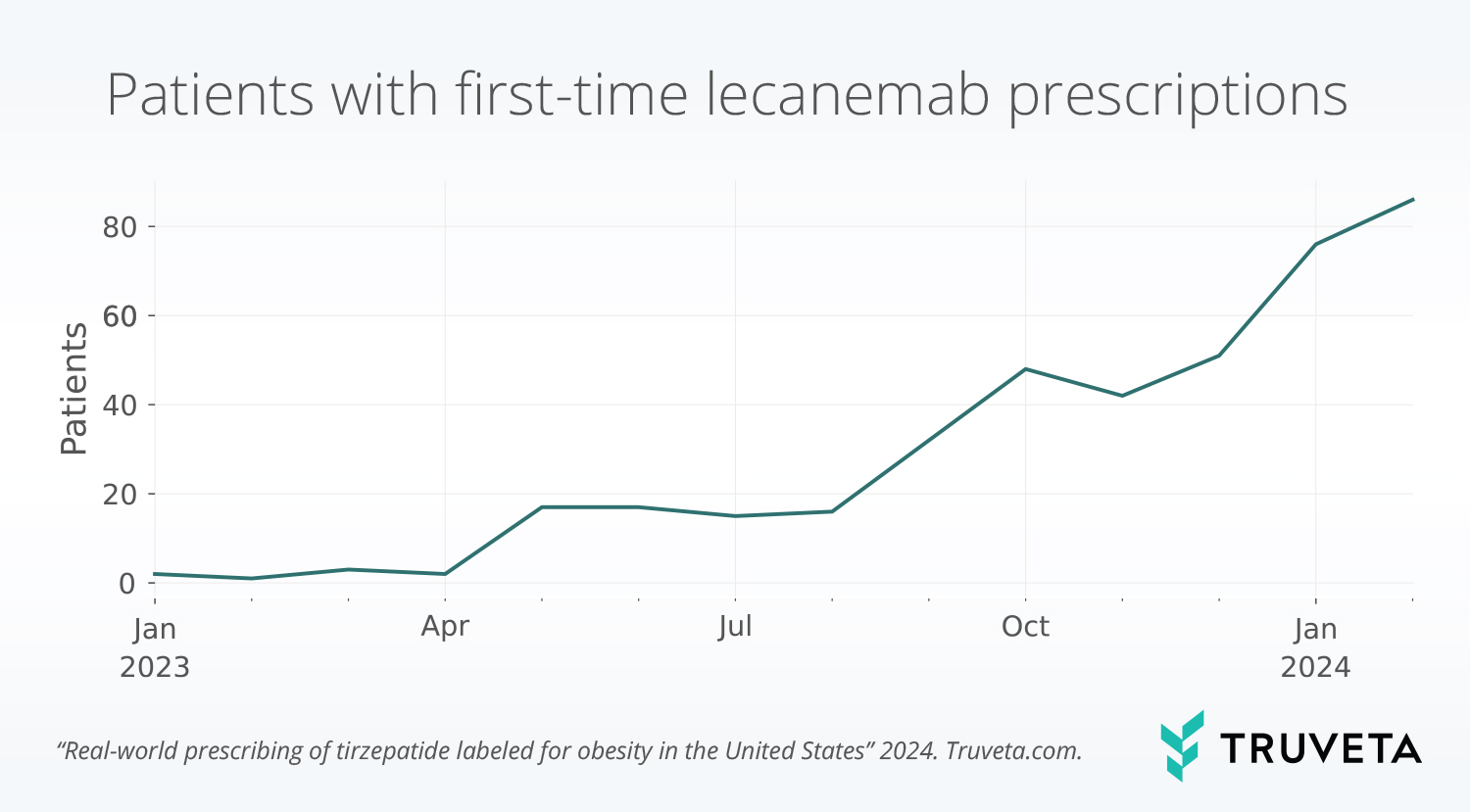
Lecanemab uptake patterns
The majority of the population had a prescription of lecanemab after the FDA granted full approval in July 2023 (89.7%). First-time lecanemab prescriptions continue to increase; February 2024 (the last month in the study period) had the most first-time prescriptions of the medication (21.1% of all prescriptions).
Discussion
Although first-time prescriptions of lecanemab continue to increase compared to patients with first-time Alzheimer’s diagnosis, the uptake remains limited. The slow rollout could be influenced by physicians refusing to prescribe the drug due to a lack of perceived clinical benefits, patients deciding the risks outweigh the benefits, or a lack of ability to get access to lecanemab due to high costs, regional availability, or other factors (Weisman, 2024). Further, as prescriptions increase, specialized dementia centers who treat Alzheimer’s patients may not be able to support the increased demand (Weisman, 2024).
On top of the slower rollout, the data here suggests there may already be disparities in patients’ ability to access lecanemab. Significant differences in the age group, race, and ethnicity of people with lecanemab prescriptions and first-time Alzheimer’s diagnoses during the study period were seen. The population with lecanemab prescriptions is made up of a larger percentage of patients who are white and younger compared to the population with Alzheimer’s. The population with lecanemab prescriptions also had higher individual incomes and increased housing stability, and a higher percentage of the population is married. Even with the small uptake, this may represent barriers to access and disparities that already exist.
There are a few limitations with this analysis. First, we used lecanemab prescriptions to describe when a person had a claim, administration, or prescription for lecanemab, even if they only had evidence of one of these. Second, we only studied first-time prescriptions; subsequent prescriptions or administrations of lecanemab are not included in this analysis. Despite these limitations, to the best of our knowledge, these are the first data to show demographic and SDOH distributions associated with the uptake of lecanemab.
These findings are consistent with data accessed on April 12, 2024.
You can also view this study directly in Truveta Studio.
Citations
Alzheimer’s Association. (2024). Lecanemab Approved for Treatment of Early Alzheimer’s Disease. https://www.alz.org/alzheimers-dementia/treatments/lecanemab-leqembi#:~:text=How%20much%20will%20this%20drug,for%20financial%20information%20and%20support
Institute for Clinical and Economic Review. (2023). ICER Publishes Final Evidence Report on Lecanemab for Alzheimer’s Disease. https://icer.org/news-insights/press-releases/icer-publishes-final-evidence-report-on-lecanemab-for-alzheimers-disease/
US Food & Drug Administration. (2023a). FDA Grants Accelerated Approval for Alzheimer’s Disease Treatment. U.S. Food & Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-disease-treatment
US Food & Drug Administration. (2023b, July 6). FDA Converts Novel Alzheimer’s Disease Treatment to Traditional Approval. https://www.fda.gov/news-events/press-announcements/fda-converts-novel-alzheimers-disease-treatment-traditional-approval
Weisman, D. (2024, January 29). Rising Leqembi Prescriptions Are Straining Clinic Capacity. https://www.alzforum.org/news/research-news/rising-leqembi-prescriptions-are-straining-clinic-capacity