New results released this week from the SURMOUNT-5 clinical trial comparing tirzepatide (Zepbound) to semaglutide (Wegovy) are closely aligned to Truveta Research’s GLP-1 comparative effectiveness study, initially shared more than a year ago on November 27, 2023 and published earlier this year in JAMA Internal Medicine.
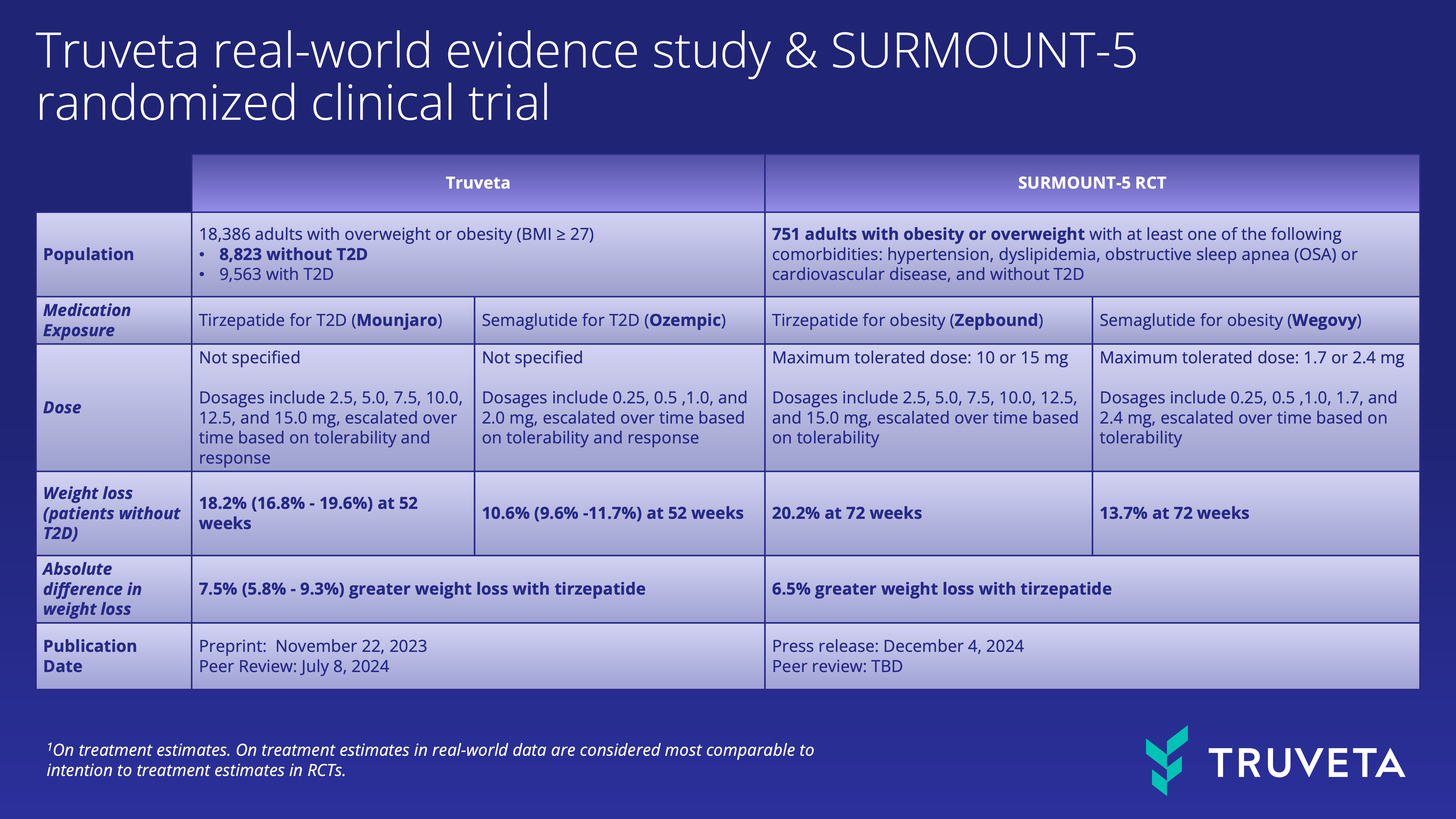
Truveta Research was able to use real-world data to explore a larger, more diverse patient population more than 10 times the size of the SURMOUNT-5 study (8,823 patients with overweight or obesity and without type 2 diabetes vs. 751). Both studies explored the comparative effectiveness of semaglutide and tirzepatide for weight loss among patients with overweight or obesity.
This example shows the power of real-world data to unlock timely insights exponentially faster and with larger, more diverse populations than previously possible by traditional methods.
Clinical trials are often limited in the size and diversity of patients able to participate. That’s why real-world data can be so much faster and more representative – because the data shows what’s actually happening with patients in real-time and not reliant on trial enrollment. It’s also challenging to know how outcomes – in this case, weight loss – observed in randomized controlled trials will generalize to real-world populations. Real-world studies can help evaluate medication effectiveness in complex care environments for large and diverse populations.
“It’s thrilling to see how closely the trial results match those in Truveta Data, despite some differences in study design,” said Tricia Rodriguez, PhD, principal applied research scientist, Truveta. “It’s also exciting to know that with real-world data, we were able to provide a glimpse into these clinical trial results over a year sooner. It helps to build confidence in the use of regulatory-grade, real-world data for scientifically rigorous research.”
Comparing the results of SURMOUNT-5 with Truveta Research’s real-world data study
As expected, there were differences in design between the two studies. First, Truveta’s study focused on the semaglutide and tirzepatide FDA-approved anti-diabetic medications (ADM; Ozempic and Mounjaro, respectively), compared with the semaglutide and tirzepatide approved as anti-obesity medications (AOM; Wegovy and Zepbound, respectively) studied in SURMOUNT-5. The dosage for AOM versions can be higher than the ADM versions, especially for semaglutide. Zepbound had not yet been approved during the study timeframe for the Truveta Research study. Second, Truveta’s study considered outcomes at 52 weeks, while SURMOUNT-5 used 72 weeks.
Despite the different doses of semaglutide and tirzepatide and timepoints studied, the results of SURMOUNT-5 and the Truveta Research study were consistent, namely that tirzepatide-based medications performed better than semaglutide-based medications:
- SURMOUNT-5 study: At 72 weeks, weight loss was 20.2% for Zepbound (tirzepatide AOM) compared to 13.7% for Wegovy (semaglutide AOM).
- Truveta Research study: At 52 weeks, weight loss was 18.2% for Mounjaro (tirzepatide ADM) compared to 10.6% for Ozempic (semaglutide ADM) for patients without type 2 diabetes (T2D)
The lower percentages in the Truveta Research study likely reflect a shorter timeframe (52 weeks instead of 72) and the use of ADM versions, which can have lower doses. Still, the absolute difference in weight change between tirzepatide and semaglutide in SURMOUNT-5 (6.5% difference at 72 weeks) falls within the confidence interval of Truveta Research’s study (7.5% [5.8% – 9.3%] at 52 weeks for ADM versions) for patients without T2D.
The widespread off-label use of ADM semaglutide (Ozempic) and tirzepatide (Mounjaro) by patients without T2D allowed Truveta Research to study comparative outcomes for a large, real-world population before clinical trials results in a similar population were available. The broadly consistent findings highlight the potential of real-world data to produce results consistent with gold-standard studies.
Value of both clinical trials and real-world data studies
Both the SURMOUNT-5 clinical trial and Truveta Research’s real-world evidence study reinforce the same key trend: tirzepatide-based medications consistently outperform semaglutide-based medications for weight loss in patients with overweight or obesity. While the specific magnitudes of weight loss differed due to methodological differences, these findings highlight the complementary value of both clinical trials and real-world data. Together, they provide a more robust understanding of medication effectiveness, with real-world data offering the most timely insights and greater population diversity, while clinical trials ensure controlled validation.
These studies are just one example of how randomized clinical trials and real-world evidence can work hand in hand to provide the critical knowledge required to advance patient care. To enable scientifically rigorous research, researchers need the best possible data.
Truveta Data, the most complete, timely, and clean electronic health record (EHR) data, includes more than 120 million de-identified patients – more than one-third of the U.S. population – empowering researchers to study patients representative of the diversity of the US.
With this representative, timely data, we look forward to partnering with more researchers to build representative and accurate real-world studies to power important real-world evidence and complement the insights found in clinical trials.