The need for better cardiovascular device insights
Medical device companies are at the forefront of innovation, but a lack of real-world clinical data (RWD) hampers progress in studying cardiovascular device performance. Traditional data sources, such as claims and hospital chargemasters, fail to capture device-specific details, patient outcomes, and longitudinal care journeys—critical elements for assessing device safety and effectiveness.
Enter Truveta Data: a transformative approach to cardiovascular device research, providing the most representative, timely, and complete patient journey data. This regulatory-grade EHR dataset – including integrated notes and images – is linked with SDOH, mortality, and closed claims to deliver unprecedented clinical depth. With daily updates and real-world clinical data, Truveta enables device manufacturers to monitor safety, compare effectiveness, and conduct regulatory-grade research with confidence.
Why medical device companies need Truveta Data
Comprehensive & diverse real-world data
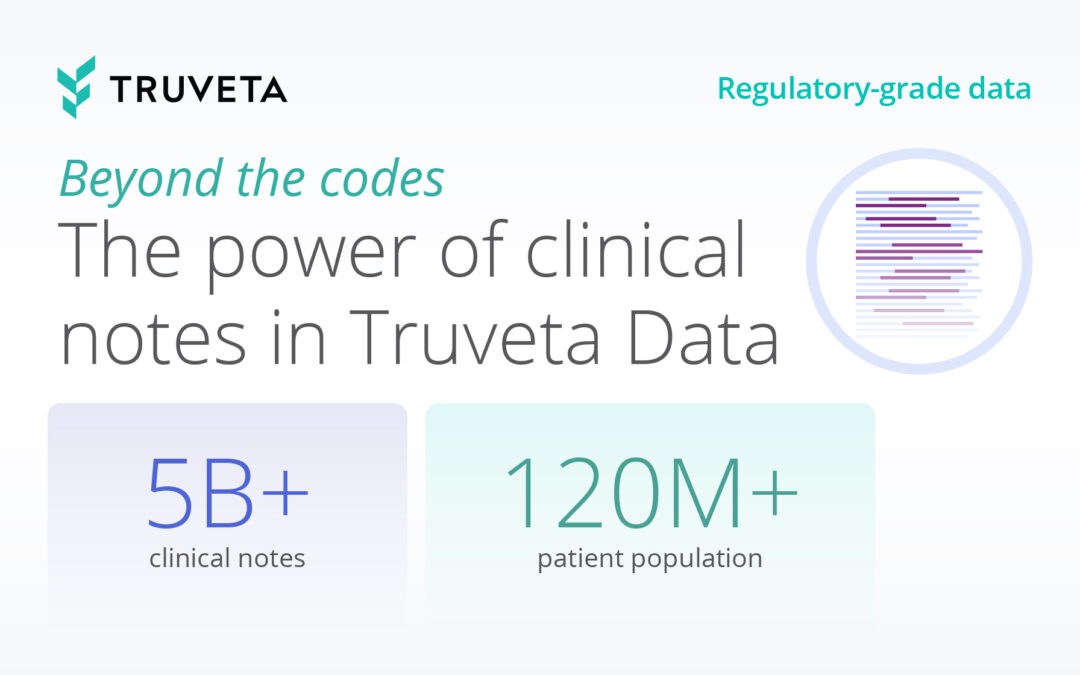
Truveta delivers EHR data from 120 million+ patients across more than 30 US health systems, covering 150,000 unique medical devices and nearly 13,000 brands. This unparalleled scale ensures insights into device performance across real-world patient populations, including underrepresented groups. Truveta Data also includes second-by-second details from operative reports, including sites and mechanisms of placement, off-label use, and immediate outcomes.
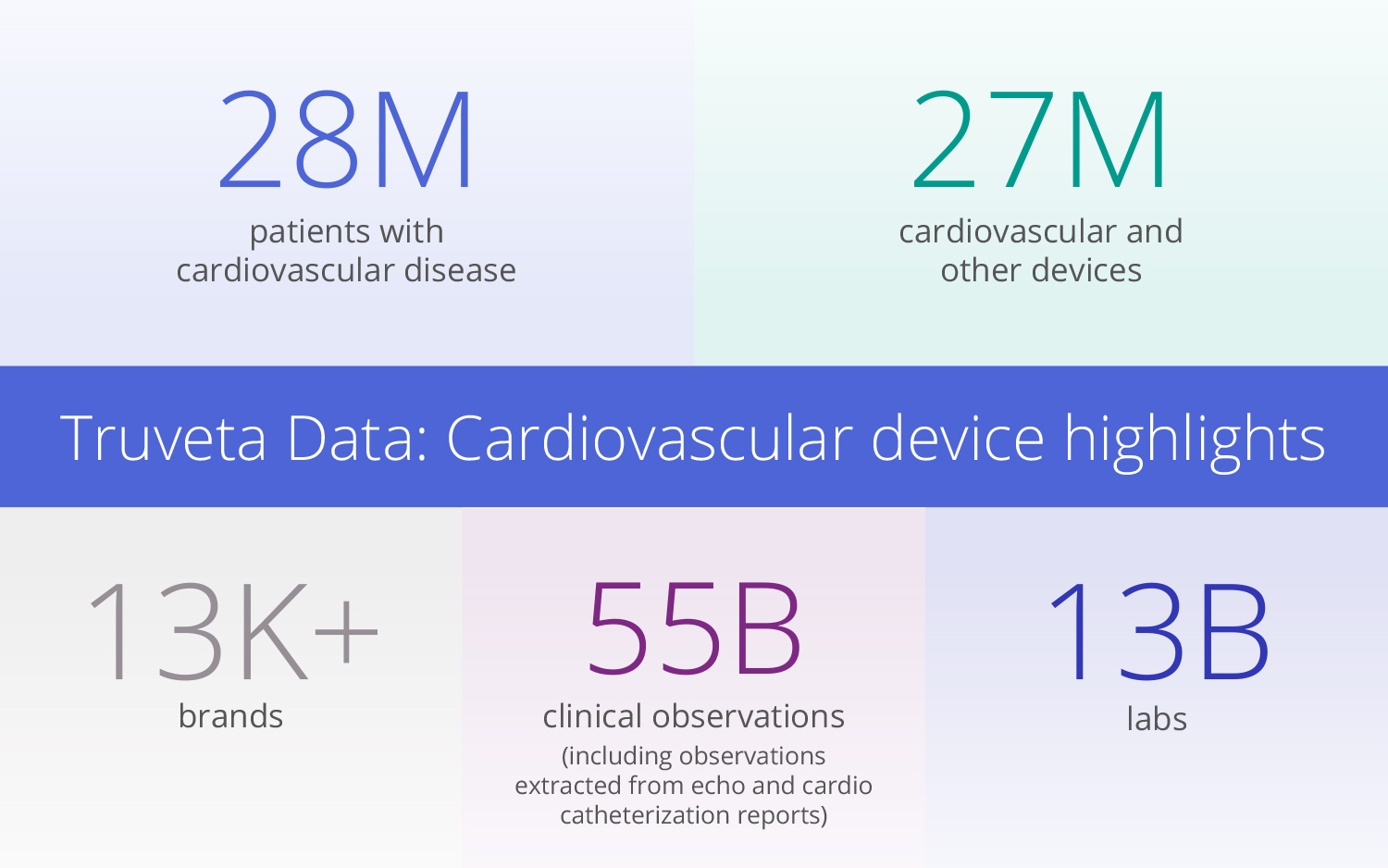
AI-driven clinical data extraction
Truveta’s AI-powered Truveta Language Model (TLM) extracts data from unstructured clinician notes, capturing key cardiovascular device measures like procedural outcomes and safety signals that are often missing from traditional databases.
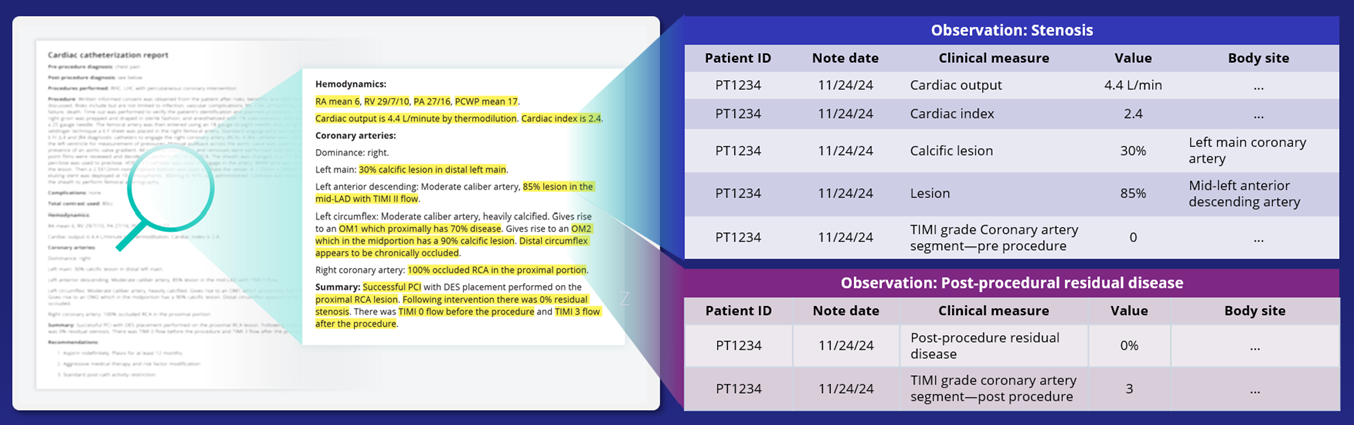
Thanks to the Truveta Language Model, researchers can also analyze:
- Over 69 million distinct cardiac measurements from 3.4 million echocardiograms
- Over 4.7 million measurements from 700,000+ cardiac catheterization reports
From essential metrics like ejection fraction to specialized measures such as tricuspid annular systolic velocity (TASV), access to these measures at scale enables researchers to explore new frontiers in their cardiovascular studies.
Daily updated insights
Unlike traditional claims-based datasets that lag by 6-12 months, Truveta’s daily data updates allow faster detection of safety signals, effectiveness trends, and disparities in device use.
Regulatory-grade data
Truveta offers regulatory and audit capabilities to facilitate real-world evidence (RWE) submissions in alignment with FDA guidance, supporting:
- Post-market surveillance
- Label expansions
- Regulatory submissions
Truveta also provides regulatory professional services.
Key areas of cardiovascular device research enabled by Truveta
The following examples represent findings from Truveta Research.
Safety signal detection and post-market surveillance
- Aortic stenosis (TAVR vs. SAVR)
- Patients in urban areas receive TAVR faster than those in rural areas, highlighting healthcare access disparities.
- Higher-income patients experience shorter wait times for treatment.
- These insights help manufacturers refine safety profiles and patient selection criteria for aortic valve devices.
Comparative effectiveness and outcomes research
- PAD treatment disparities
- Black and Hispanic patients undergo revascularization at lower rates than white patients and receive drug-eluting stents less frequently, despite evidence supporting better outcomes.
- Patients treated with bare-metal stents and balloons face higher rates of amputation and readmission within a year.
- These findings can inform device positioning, guideline updates, and reimbursement strategies.
Patient subpopulation insights
- Underdiagnosed PAD in younger patients
- 6.6% of PAD patients are under 50, yet research in this group is scarce.
- Black and Hispanic patients are disproportionately affected, suggesting earlier disease onset and potential gaps in screening and care.
Device durability and long-term outcomes
- Tracking TAVR vs. SAVR longevity
- Using Truveta Data, researchers classified aortic stenosis severity using echocardiograms, allowing precise tracking of long-term valve durability and complications.
AI and imaging analysis for cardiovascular devices
- Extracting ejection fraction data for heart failure research
- The Truveta Language Model extracts quantitative EF values from echocardiogram reports, improving device efficacy assessments.
- 3.4 million echocardiogram reports available for large-scale cardiovascular studies.
Economic and market intelligence
- PAD diagnosis trends in the US
- PAD testing rates doubled from 2018-2023 but declined in 2024.
- Device manufacturers can use these trends to anticipate market demand and refine outreach strategies.
The competitive advantage of partnering with Truveta
Regulatory Readiness
- Strengthen regulatory submissions with FDA-aligned RWE
- Detect safety signals earlier to avoid costly recalls
- Enable audit readiness with full provenance of data sources
Market Differentiation
- Use real-world insights to showcase superior device performance
- Support payer negotiations and reimbursement strategies
- Uncover diagnostic and therapeutic context for outcomes with clinical notes
- Generate more compelling RWE with imaging integrated with complete EHR data
Faster Innovation
- Research-ready data including unique device identifiers without fuzzy matching
- Real-time data accelerates device development cycles
- Enables proactive risk mitigation and faster market entry
- AI-ready data structures purpose-built for model development
Leverage Truveta Data for your next cardiovascular device study
With Truveta Data, medical device companies gain a strategic edge—unparalleled clinical depth, real-time data, and the ability to improve device safety, efficacy, and market adoption.
Discover how Truveta can transform your research. Contact us today for a demo and see the power of real-world cardiovascular data firsthand.