Truveta Studio
Accelerate scientifically rigorous research with immediate access to Truveta Data
Clinical research has been slowed by data inaccessibility, challenges analyzing large datasets, and opaque methodologies.
Accelerate scientifically rigorous research with immediate access to Truveta Data, powerful analytics, and AI.
Immediate access to Truveta Data

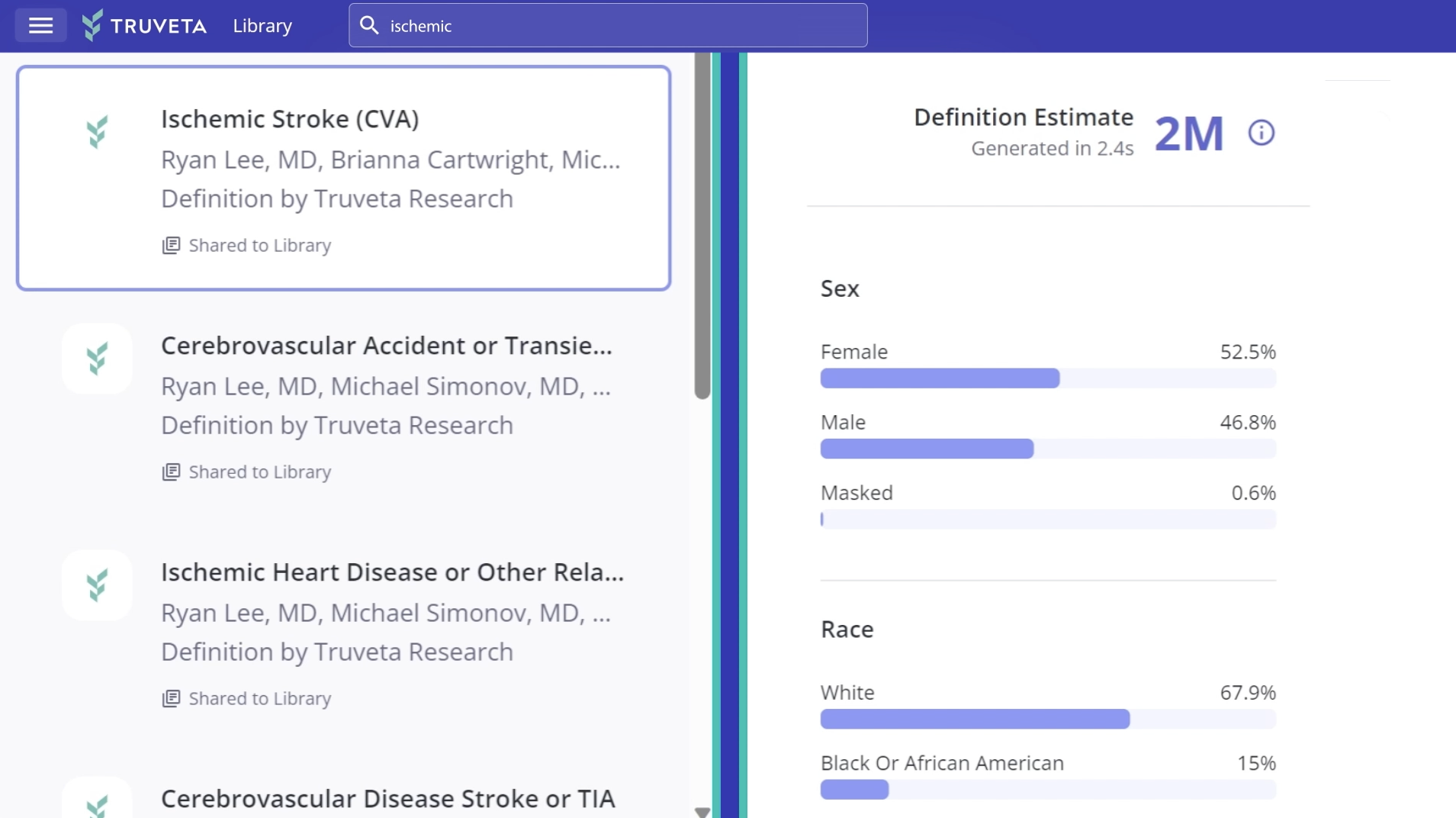
Quickly study complete EHR data, including notes and images, for more than 120 million patients linked with closed claims data, including economics, for more than 200 million patients.



Gain real-time insights with daily updated data eliminating delays and gaps from static, stale data cuts.

Quickly study complete EHR data, including notes and images, for more than 120 million patients linked with closed claims data, including economics, for more than 200 million patients.



Powerful analytics and AI

Combine EHR, closed claims, and multiomic datasets into feature tables within integrated scalable notebooks supporting medical statistics and visualization libraries.

Leverage Truveta Library with thousands of clinical definitions and study templates to accelerate time to insight. Share content within the Library to facilitate team productivity and peer review.

Ask Truveta Tru for natural language assistance to develop hypotheses and discover trends through iterative prompts and data visualization.
Regulatory grade evidence generation



Click to enlarge
Click to enlarge



Trusted research environment is audited for HITRUST r2, SOC 2 Type 2, and ISO 27001 security and privacy compliance.
Tru: Natural language research assistance, powered by generative AI

Accelerate research with Tru, trained on the Truveta Language Model and Truveta Data.

Build and visualize patient populations quickly and get started with medical code sets.

Develop hypotheses and discover trends through iterative prompts and data visualizations.

Access source information and underlying code sets behind responses.
Explore research conducted using Truveta Studio


Analytics flexibility to support your needs

Easily share studies with others inside or outside your organization.

Grant external research partners direct access to Truveta Studio, fostering seamless collaboration in a trusted, secure environment.

Export Truveta Data into your own computing environment, including SAS and Excel, or work with one of our preferred analytics partners.
Trusted by leading life science, public health, and healthcare organizations
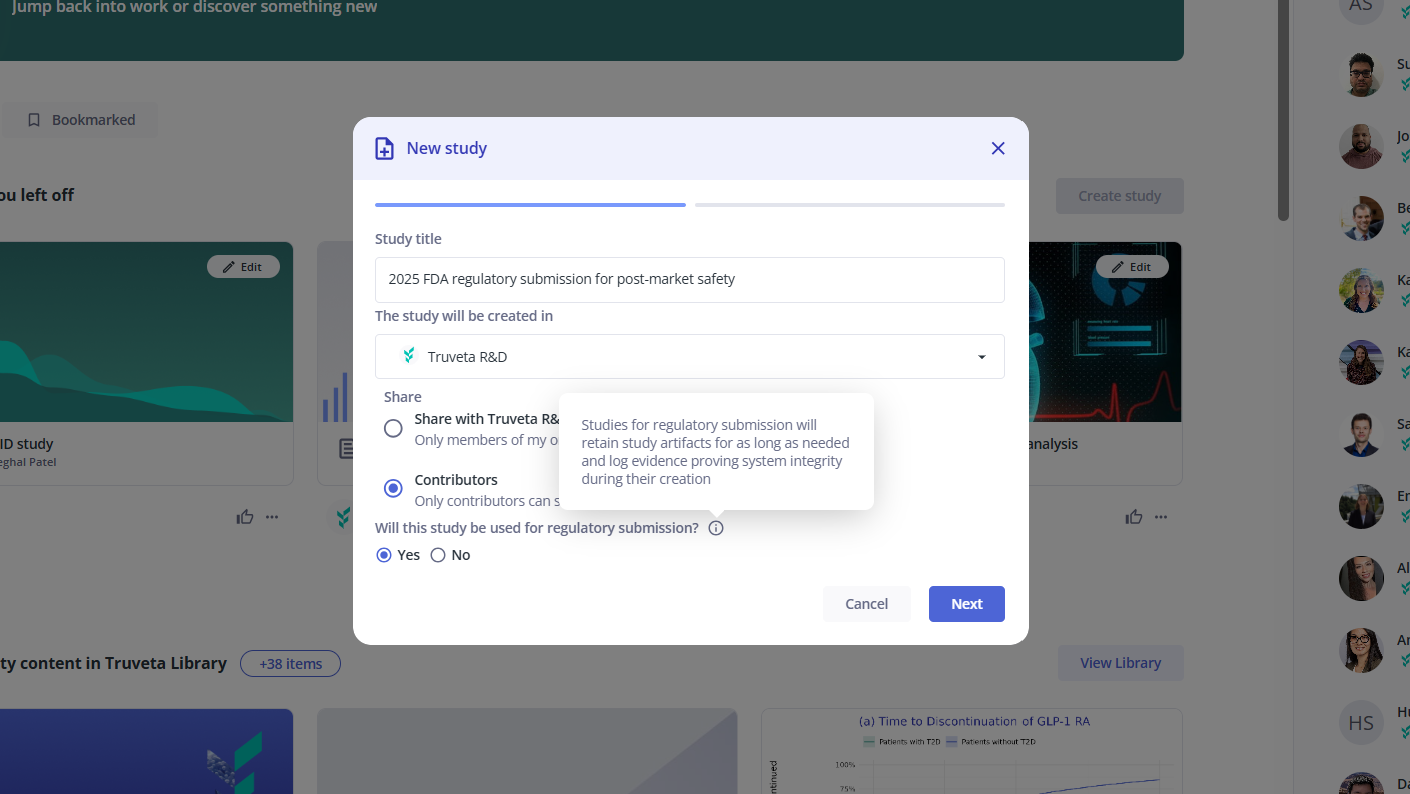
Generate evidence for regulatory submission
Truveta has invested in policies, procedures, and safeguards, enabling researchers to confidently submit evidence generated using Truveta Studio to the FDA for regulatory review.
Example: Screenshot showing how a researcher would designate a study as intended for regulatory submission to yield verifiable evidence of data quality and system integrity at the time of analysis.