
by Truveta staff | Sep 12, 2025 | Data
Non–small cell lung cancer (NSCLC) accounts for about 85% of lung cancer cases and remains the leading cause of cancer death worldwide. While major advances in targeted therapies and immunotherapies have transformed care, gaps persist in biomarker testing, treatment...

by Truveta staff | Aug 25, 2025 | Data
Multiple sclerosis (MS) affects approximately 1 million people in the United States, with women representing nearly 75% of patients. Over the past decade, consensus guidelines from leading MS experts have increasingly emphasized the importance of early intervention...

by Truveta staff | Aug 18, 2025 | Data
After years of uncertainty, a recent policy change is sparking new optimism in rare disease drug development. A change in federal policy now keeps certain multi-indication rare therapies exempt from Medicare price negotiations—a rule that had discouraged some...

by Truveta staff | Aug 4, 2025 | Data
Why do patients stop taking a medication? Was it due to side effects, access barriers, or lack of efficacy? These are not just clinical questions; they’re strategic ones. Understanding treatment discontinuation is essential for improving therapy design, informing...

by Truveta staff | Jun 19, 2025 | Data
In May 2025, the FDA cleared the first blood test to aid in diagnosing Alzheimer’s disease, making early detection more accessible than ever. The Lumipulse test measures biomarkers in the blood associated with Alzheimer’s pathology, offering a simpler alternative to...
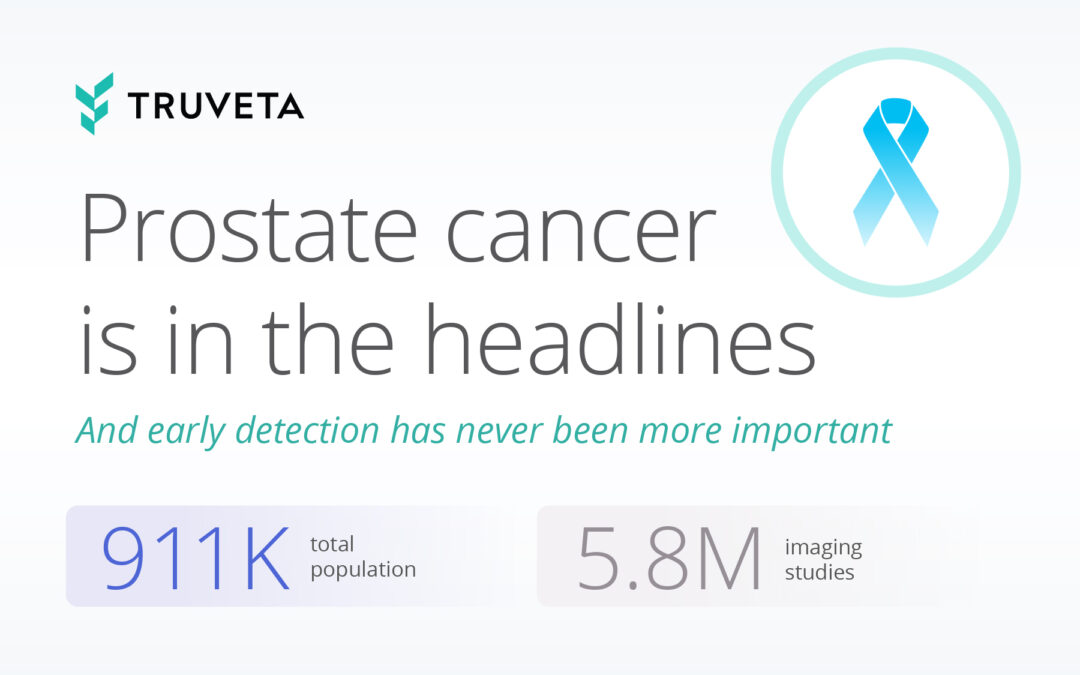
by Truveta staff | May 29, 2025 | Data
Each year, more than 34,000 men in the U.S. die from prostate cancer. While early-stage disease is often highly treatable, outcomes still vary widely — especially by race, income, and geography. Catching the disease early can save lives, but real-world data on...







