
by Truveta staff | Jun 17, 2024 | Data, News, Technology
Truveta now offers regulatory and audit capabilities to support our customers for real-world evidence (RWE) submissions to the Food and Drug Administration (FDA) and other global regulatory authority decisions. These regulatory grade capabilities advance Truveta’s...
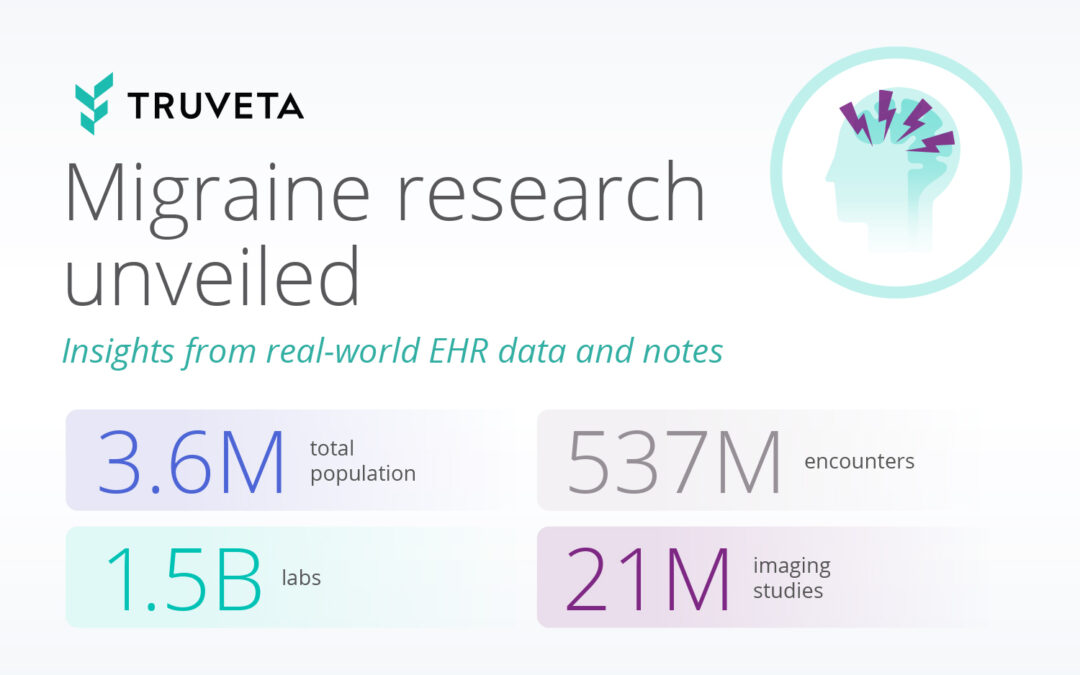
by Truveta staff | Jun 14, 2024 | Data
One in every four US households has someone living with migraine. More than a severe headache, migraine is a complex neurological disorder impacting 39 million Americans with more than $36B lost each year in medical expenses and lost productivity. Despite its...
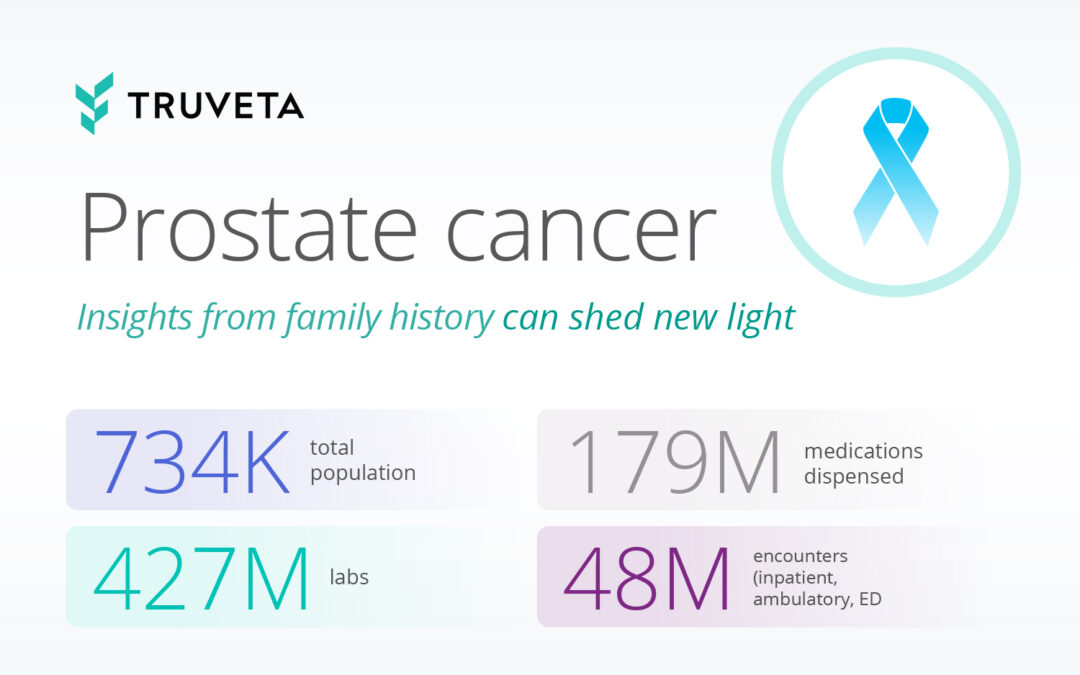
by Truveta staff | May 20, 2024 | Data
You probably know someone who will be diagnosed with prostate cancer in their lifetime. 1 in 8 men will develop prostate cancer, with a higher risk in Black and African American men and an overall increased risk after the age of 65. While most cases are not fatal,...

by Truveta staff | May 1, 2024 | Data, News, Technology
Today we are excited to announce the availability of expanded concepts from clinical notes — including family history, medication details reported to providers, and complex concepts for a wide range of therapeutic areas from cardiology to rare disease and more –...

by Truveta staff | Apr 25, 2024 | Data, News
Today, we are excited to announce the largest and most complete mother-child electronic health record (EHR) dataset for scientifically rigorous research on mothers and their children. Truveta empowers researchers with unparalleled insights into the continuum of care...
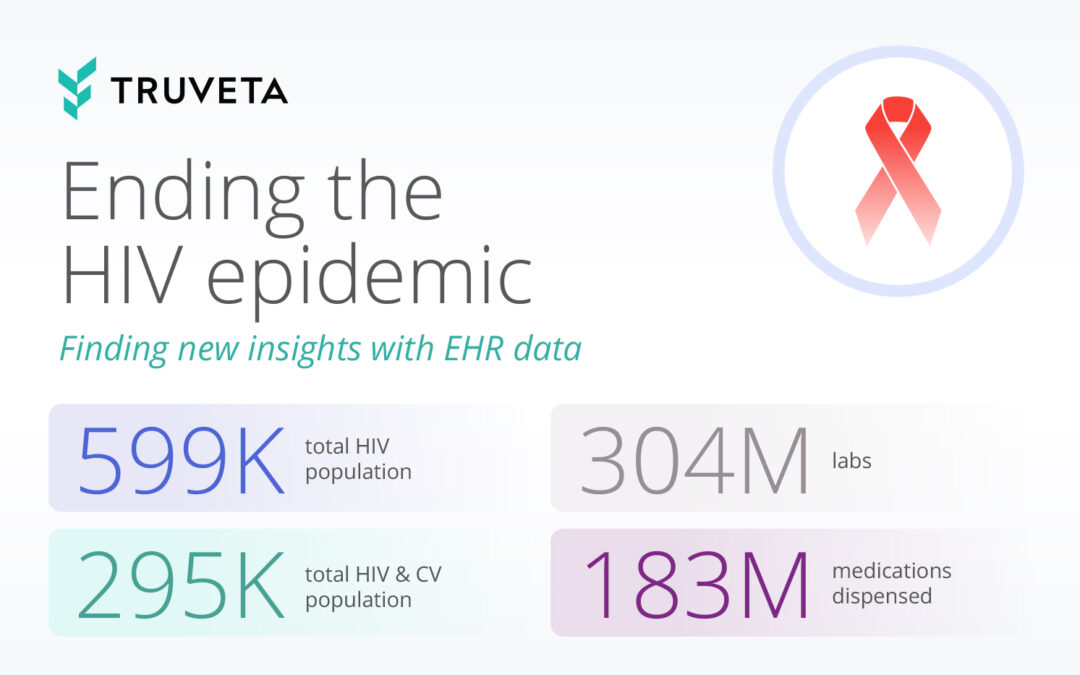
by Truveta staff | Apr 18, 2024 | Data
In 2019, the US government unveiled the ambitious Ending the HIV Epidemic (EHE) plan, aiming to slash new HIV infections by 90% by 2030. Thanks to advances in antiretroviral therapy (ART), proven models of prevention, and data access, new HIV infections declined 12%...







