“For the first time in the history of health, we have enough data at scale to dramatically advance innovation in healthcare” – Michael Slubowski, president & CEO, Trinity Health
Electronic health record (EHR) data is rapidly changing how life sciences, pharmaceutical, and public health organizations monitor and respond to respiratory viruses such as influenza, RSV, and COVID-19. It was, in fact, the COVID-19 pandemic that served as the catalyst to bring health systems across the country together to form Truveta. The world needed faster answers– and a much larger dataset to statistically serve all patients.
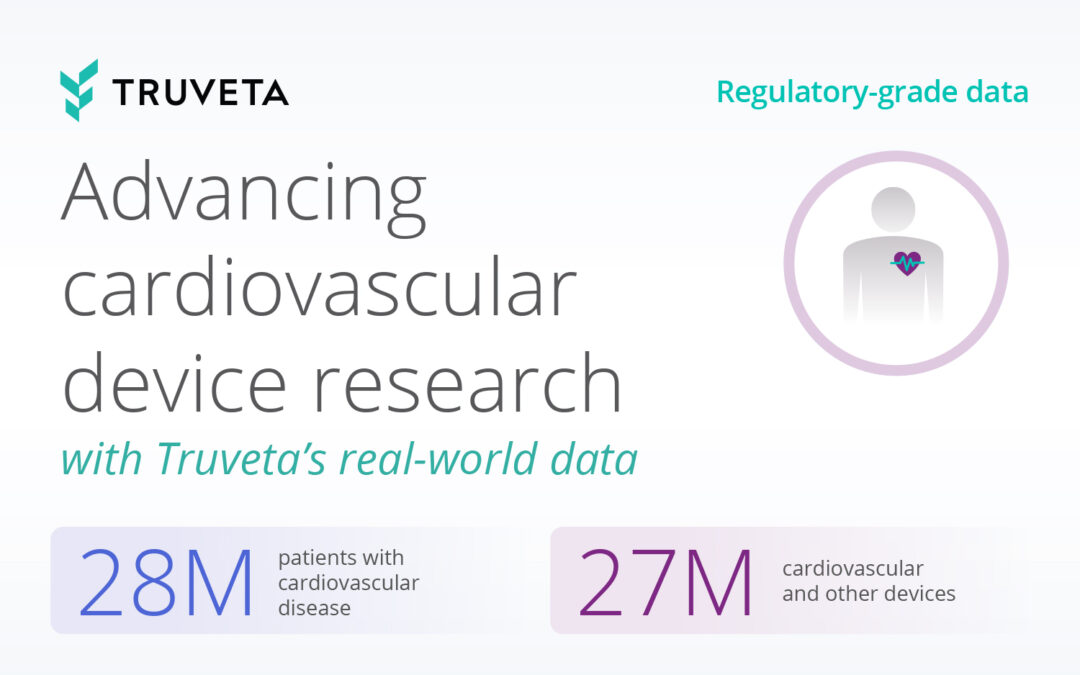
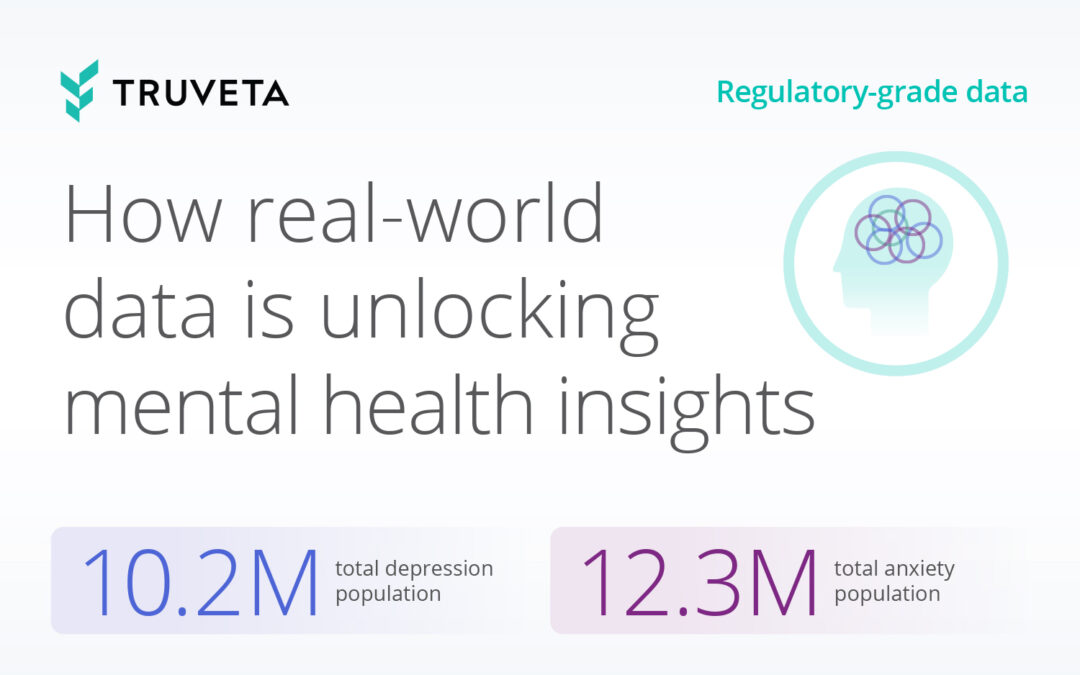
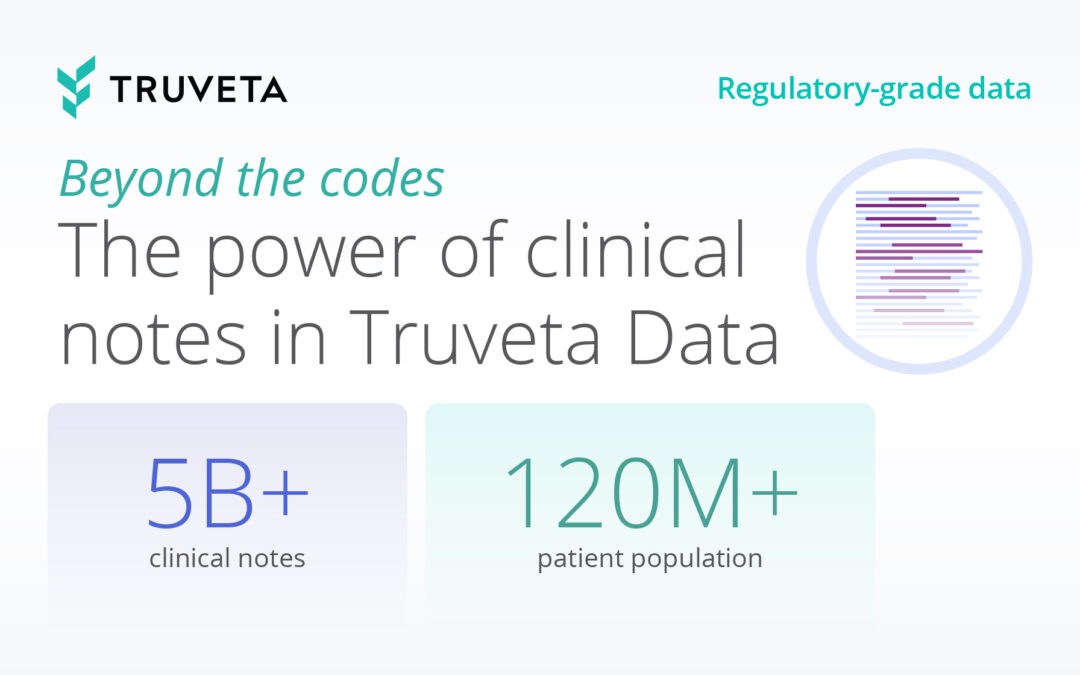
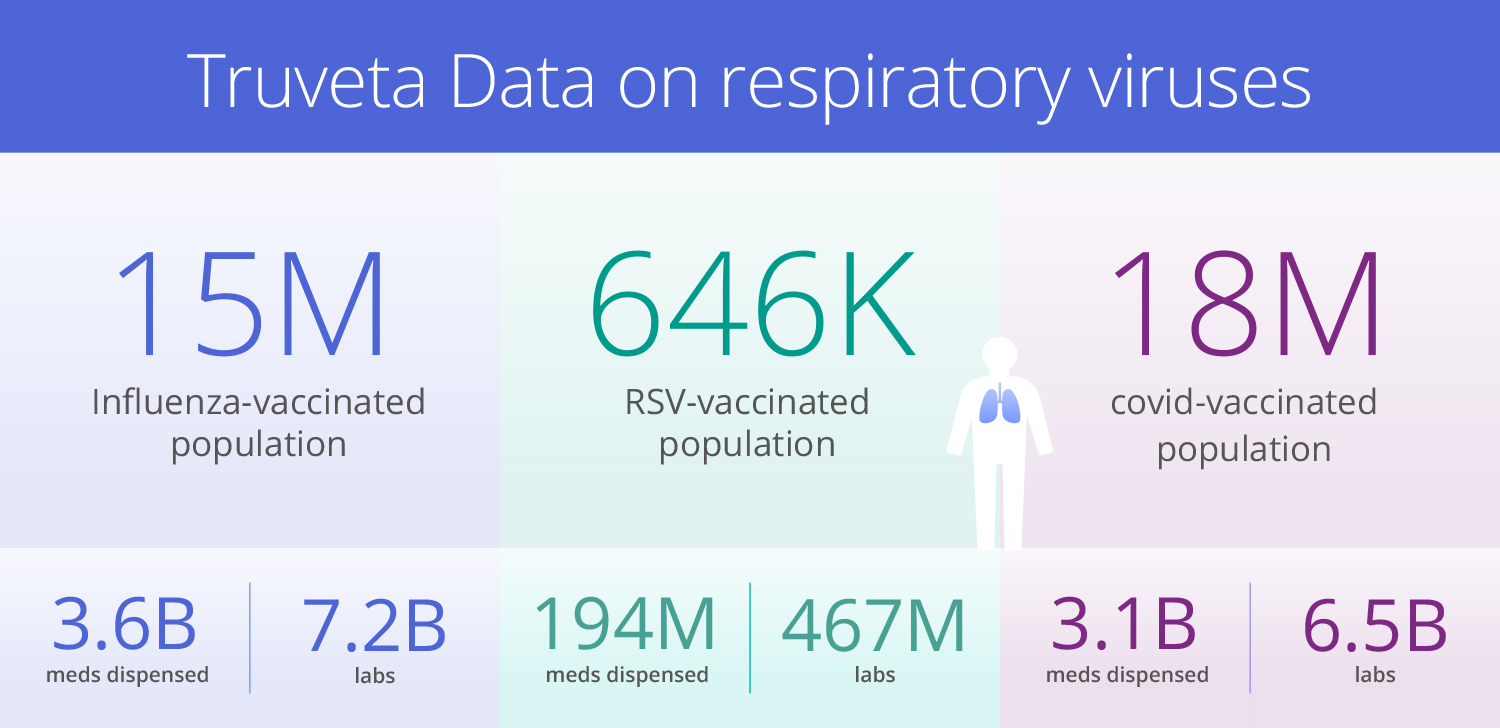
Today, with over 120 million de-identified patients and 30 member health systems, Truveta is trusted by leading life science, government, academic, and healthcare organizations to monitor and study infectious diseases impacting public health. Truveta Data delivers the most complete EHR data – including clinical notes and images – linked with SDOH, mortality, and claims data. This regulatory-grade data enables researchers to:
- Monitor virus trends and vaccine safety with daily updated data.
- Analyze the impact of SDOH variables on healthcare access and outcomes.
- Gauge the impact of policies on health outcomes, healthcare utilization, costs, and population health with complete EHR data linked to SDOH, mortality, and claims data.
- Study maternal and pediatric health with access to more than 1 million mother-child pairs.
Read on to explore key respiratory virus use cases and the critical data elements that empower actionable insights, enhance vaccine strategies, and improve patient outcomes.
1. Real-time epidemiological surveillance
Daily updated EHR data is indispensable for tracking disease trends, seasonality, and the effectiveness of public health interventions down to state and zip code levels. This granular breakdown provides insights into disease spread and severity, enabling tailored interventions. Filtering by specialist type—such as pulmonologist or pediatrician—helps researchers focus on data most relevant to respiratory illnesses.
Key data elements: Hospitalizations, virus testing, geographic breakdown by state, zip code, provider type
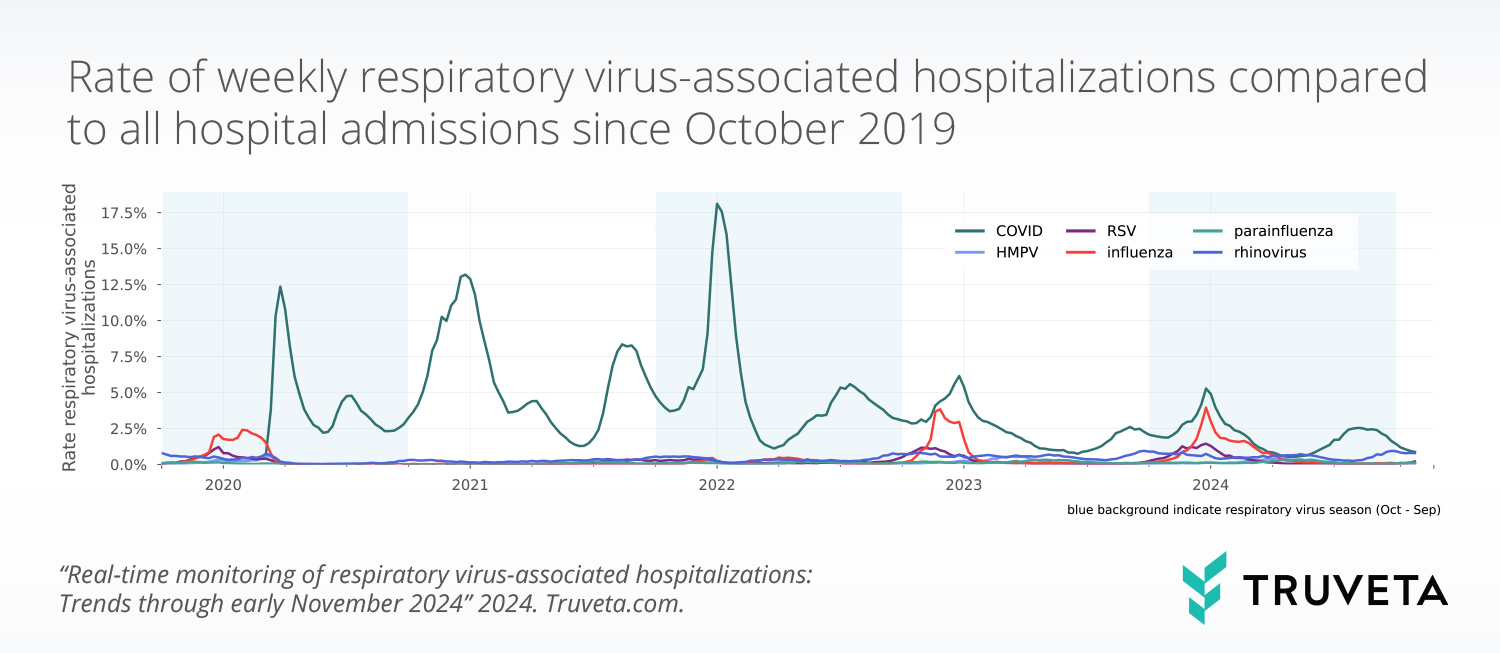
Truveta Research has created a monthly respiratory virus monitoring report based on Truveta Data to supplement the surveillance data provided by the CDC.
2. Vaccine efficacy and uptake analysis
Researchers can assess vaccine effectiveness and coverage rates by age group, sex, race/ethnicity, geographic area, as well as completion rates for multi-dose regimens. Insights into brand usage of vaccines provide an understanding of patient preferences and potential barriers to uptake. Truveta Research also leveraged vaccine data to analyze trends in pediatric RSV immunizations and RSV-associated hospitalizations, one of the first studies to show the low rate of RSV immunization in the eligible pediatric population.
Truveta also delivers the largest and most complete mother-child dataset, enabling better understanding of the mechanisms of maternal vaccination safety and pediatric protection.
Key data elements: Comprehensive vaccine records (including coverage rates, completion rates, brand usage), geographic/demographic data, notes, clinical outcomes, more than 1 million directly linked mother-child pairs

Example clinical note highlighting vaccine history.
3. Post-market safety surveillance of antivirals and vaccines
Timely EHR data facilitates ongoing monitoring of vaccine and antiviral drug safety and performance post-approval. Researchers can track top vaccines and antivirals prescribed to patients and assess how treatment adherence influences outcomes. By evaluating biomarkers and clinical outcomes, such as hospitalization rates, breakthrough infections, or symptom severity (available via clinical notes), researchers gain insights into real-world effectiveness and patient outcomes.
Key data elements: Real-world data on medication use, biomarkers, notes, and clinical outcomes including patient reported outcomes
4. Diagnostic development and accuracy
Accurate and rapid diagnosis is critical for managing respiratory illnesses. Complete EHR data provides clinical validation for advanced testing technologies, as well as diagnostic accuracy against real-world outcomes and biomarkers.
Medical images, such as chest x-rays or CT scans, also play a crucial role in diagnosing respiratory infections and evaluating severity. When combined with other data such as lab results, these images can confirm or refine diagnoses, identify patterns of co-infection, and aid in the development of predictive models for disease severity and outcomes.
Key data elements: Lab results, images, comorbidities, diagnostic data, treatment data
5. Understanding co-infections and clinical outcomes
Co-infections, such as COVID-19 paired with influenza or RSV, complicate disease severity and treatment outcomes. Truveta’s EHR data allows researchers to examine these interactions in depth, studying how co-infections influence hospitalization rates, ICU admissions, and mortality. With access to the full medical record across a diverse population at scale, researchers can better understand variations in outcomes by age group, underlying comorbidities, and vaccination status.
Key data elements: Hospitalization admissions, all vaccine records, mortality data, demographic/geographic trends
6. Therapeutic development, including for at-risk populations
Truveta Data supports precise targeting of treatments for at-risk populations by providing granular insights into real-world usage of medications, outcomes, and biomarkers. Researchers can evaluate how different treatments perform among vulnerable groups such as infants and older adults or those with comorbidities like asthma and COPD, demographic groups, or geographic regions. These insights allow for the development of targeted therapies that address the unique needs of high-risk populations, improving efficacy, and reducing healthcare disparities.
Key data elements: Biomarkers, medications, complete lab results, clinical outcomes, demographic data
Generate evidence with the most complete EHR data for studying infectious disease
Timely and complete EHR data is indispensable for monitoring infectious diseases and informing public health strategies. From disease surveillance to therapeutic development, the ability to analyze data elements like geographic trends, vaccination rates, and biomarkers transforms how researchers approach respiratory illnesses. With these insights, life science, pharma, and public health organizations can better protect public health, improve patient outcomes, and prepare for future challenges.
Learn more about Truveta Data and contact us for a demo today.