GLP-1 medications are revolutionizing the way we treat type 2 diabetes and obesity. First discovered forty years ago, GLP-1 receptor agonists were initially approved for type 2 diabetes in 2005, with the first weight-loss-specific approval following in 2014. Flash forward a decade, and an estimated 1 in 8 adults has tried a GLP-1 medication.
While GLP-1 prescriptions have been skyrocketing, it’s likely just the beginning. A recent study projects that if all eligible adults had access, over 50,000 deaths could be prevented annually, including more than 11,000 with type 2 diabetes. Beyond managing diabetes and obesity, emerging research is exploring GLP-1s for everything from treating addiction and lowering cardiovascular risk, to reducing knee pain. Understanding these diverse effects and optimizing GLP-1 therapies across patient populations is essential – and for this, real-world data (RWD) is key.
Why real-world data matters for GLP-1 research
Clinical trials, limited in scope and duration, cannot provide the longitudinal and representative data needed to fully capture the broad impacts of GLP-1s in real-world settings. RWD provides the depth and breadth needed to address critical questions, revealing how GLP-1s perform across medical histories, comorbidities, label expansions, and populations not studied in clinical trials (such as pregnant women).
Enter Truveta Data – the most complete, timely, and clean regulatory-grade data, with the largest collection of EHR-integrated clinical notes. Truveta Data has already powered some of the most cutting-edge research on GLP-1s, including the largest comparative effectiveness study to date of tirzepatide (Mounjaro) vs. semaglutide (Ozempic) for weight loss, published in JAMA Internal Medicine.
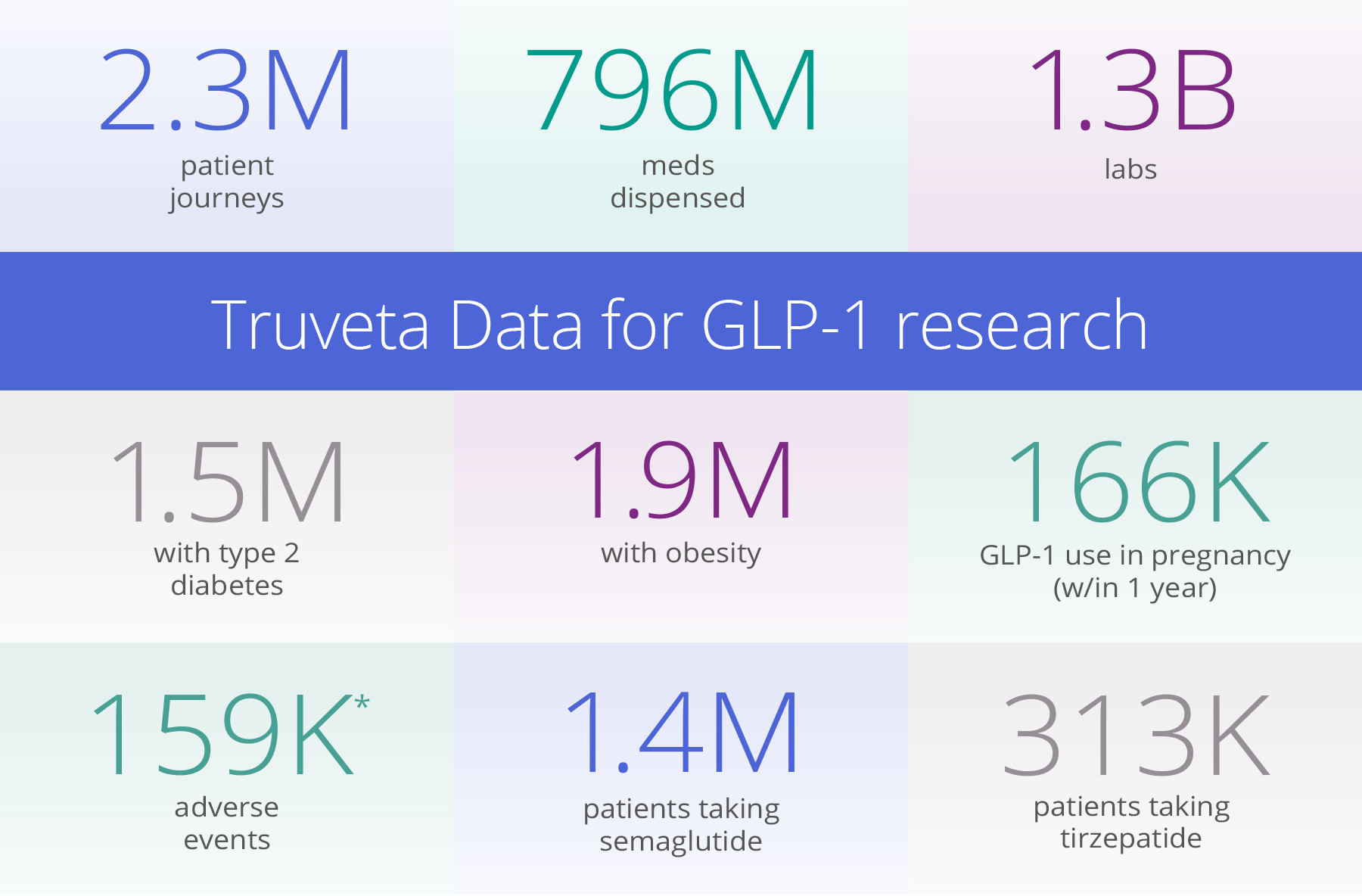
*Includes gastroparesis, cholecystitis, cholelithiasis, bowel obstruction, cyclic vomiting, syndrome, gastroenteritis, and pancreatitis.
What makes Truveta Data so impactful for GLP-1 research?
Truveta’s regulatory-grade data includes full patient medical records – including notes and images – linked with SDOH, mortality, pharmacy dispensing, and claims data for more than 100 million patients. This real-world dataset offers several unique advantages for studying GLP-1 medications:
- Timely data: Truveta Data is updated daily, providing near real-time insights critical for tracking prescribing trends, medication shortages, and emerging treatment patterns in a rapidly evolving landscape. Timely data also enables researchers to study new drugs as soon as patients begin taking them.
- Scale and representativeness: Flowing from a collective of 30 health systems, Truveta Data is representative of the full diversity of the US. This ensures researchers can study GLP-1 therapies in populations that reflect real-world diversity, accounting for variations in age, ethnicity, socioeconomic status, and comorbid conditions.
- Detailed patient-level data: Truveta Data includes biometric data (such as weights), dispense data, lab values (such as A1C levels), and SDOH. Unlike claims datasets often lacking this depth, Truveta’s EHR data captures both clinical and prescribing information.
- Comprehensive prescribing and dispensing data: Prescribing data from the EHR is linked with dispensing data, providing a more accurate view of medication use. Since GLP-1s are often not covered by insurance, comprehensive, payer-agnostic dispense data offers benefits over pharmacy claims by including patients who pay out-of-pocket.
- Concepts from notes: Truveta receives all clinical notes generated during patient care. The Truveta Language Model (TLM) then extracts data from these notes at scale, making previously unstructured data available for research. Custom note concepts could include in-depth diet/nutritional information, behavioral interventions attempted for weight loss, as well as side effects from medication use.
Use cases for GLP-1 research powered by Truveta Data
The below examples highlight some of the key use cases for studying GLP-1 data, all of which have already been enabled by Truveta Data:
- Comparative effectiveness of GLP-1 therapies: In the largest comparative effectiveness study to date on tirzepatide vs. semaglutide, Truveta Data enabled researchers to assess how these medications perform head-to-head in a large, diverse population. With a massive pipeline of GLP-1s coming to market, comparative studies will become increasingly important to differentiate products.
- Monitoring prescribing trends and shortages: Truveta’s daily-updated data allows for the monitoring of GLP-1 prescribing trends, helping researchers identify shifts in practice, as well as potential shortages of medications. This timeliness is especially important as demand for GLP-1s grows, both for approved indications and off-label use.
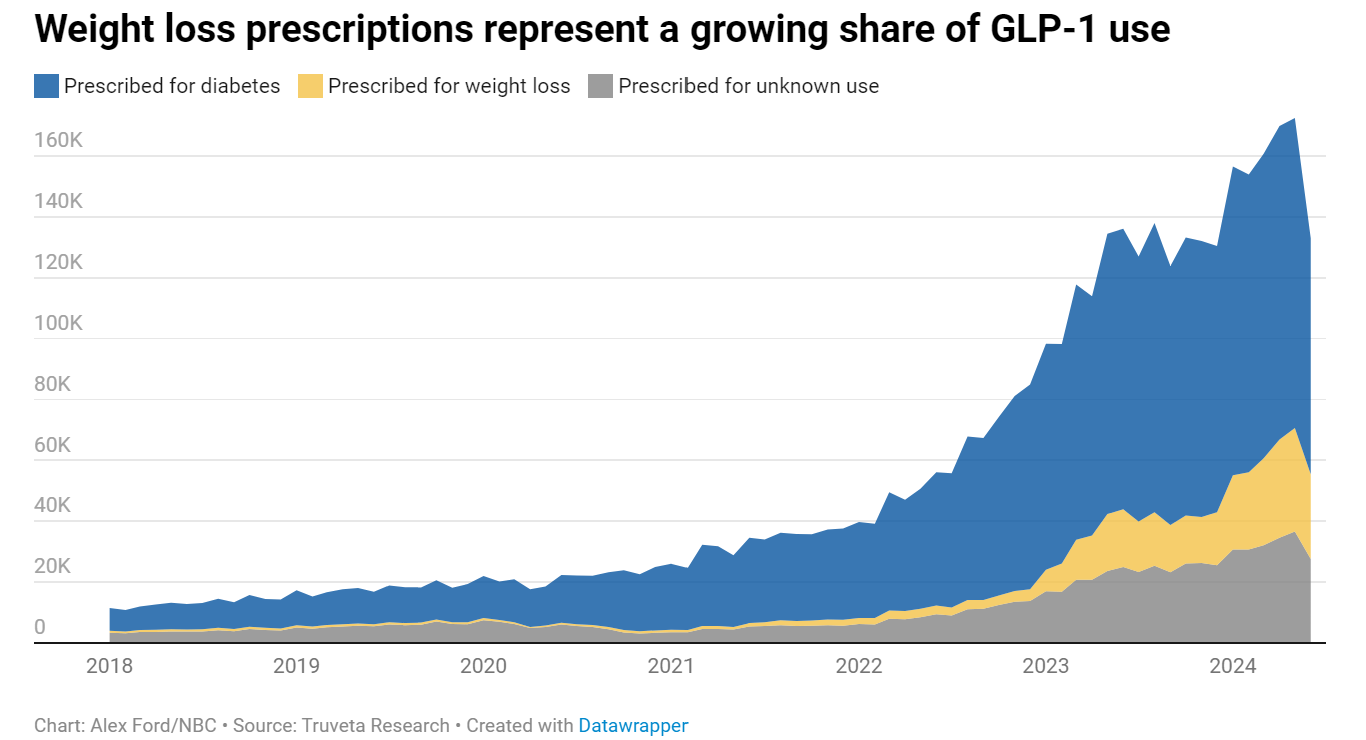
- Tracking switching between medications: Understand why and when patients switch between different GLP-1 therapies. Truveta Data captures both prescribing and dispensing information, providing rare insights into off-label use and patient decisions, including those who pay out of pocket.
- Use of continuous glucose monitors (CGMs): Many patients on GLP-1 therapies also use continuous glucose monitors (CGMs). While CGM data is often siloed, linking it with Truveta’s rich clinical data allows for more comprehensive studies on the combined effects of GLP-1s and CGMs on glycemic control, treatment adherence, and overall patient outcomes.
- Expanding access for Medicare-aged adults: Access to GLP-1 therapies for Medicare-aged adults is a growing area of research, especially as the obesity epidemic impacts older populations. Truveta Data allows researchers to study trends in treatment access, outcomes, and safety in this demographic, which is often underrepresented in clinical trials.
- Studying medication patterns: Truveta’s longitudinal patient data enables researchers to explore why patients may stop and re-start GLP-1 therapies, offering insights into adherence, side effects, and the real-world durability of these medications. Additionally, studies on youth and adolescent use of GLP-1s, another rapidly growing area of interest, are now possible with this real-world data. The latest project from Truveta Research, now available as a pre-print, explores the use of obesity treatments in youth.
The future of GLP-1 research
As GLP-1s continue to reshape the treatment of type 2 diabetes and obesity (along with an expanding list of potential indications), real-world evidence will be essential for optimizing their use, ensuring access, and tailoring treatments to the individual needs of patients.
By leveraging timely, patient-level data that captures both clinical and real-world behaviors, researchers can dive into the nuanced impacts of GLP-1 therapies across diverse populations. With the ability to monitor long-term outcomes, explore off-label use, and study real-world prescribing patterns, Truveta Data represents a transformative resource for advancing the future of GLP-1 research.
