ISPOR Europe 2023 provided Truveta an opportunity to showcase research findings, interact with life sciences leaders from across the globe, and gain insight into how different audiences view current challenges and opportunities related to real-world data (RWD).
Here are some of our key takeaways from the event.
Researchers are eager for patient-level data at scale
Gathering data of interest for a desired patient population is often time- and resource-intensive and may result in a less-than-ideal dataset. Common challenges cited by researchers across countries included fragmentation and inaccessibility, missingness, lack of baseline data, attrition, lack of standardization, and difficulty matching patients across data sources.
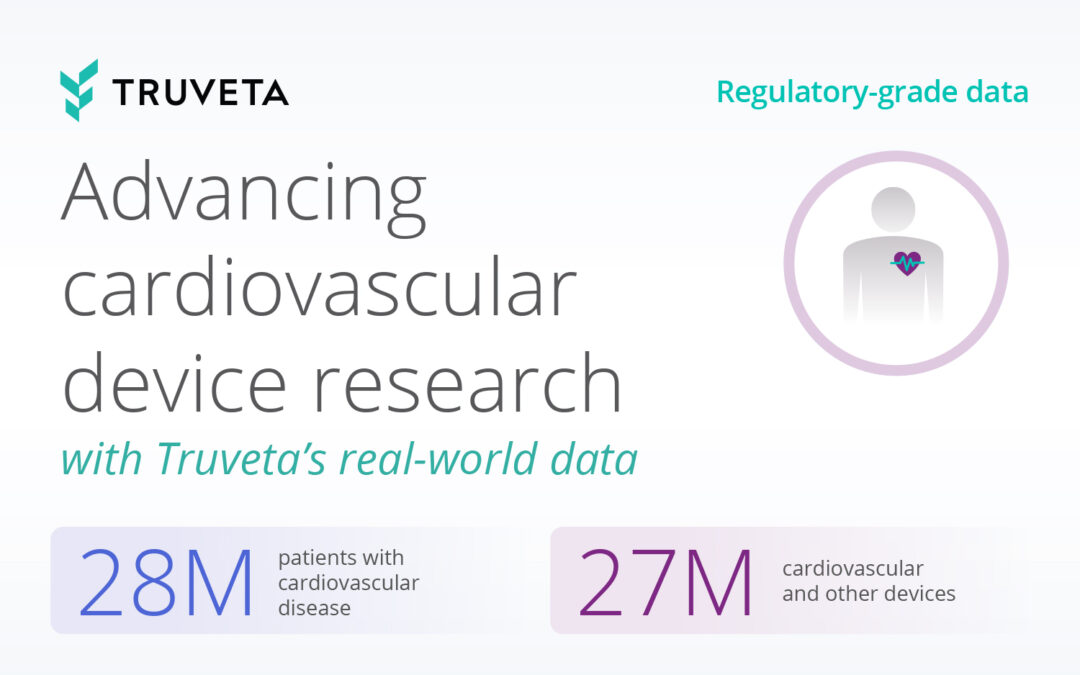
Truveta addresses these challenges by providing complete EHR data, including labs, images, and clinician notes, linked with claims and SDOH data. Truveta Data is linked across 30+ health systems, cleaned and structured for research using the Truveta Language Model. With on-demand access to a population of more than 100M patients, researchers can conduct nuanced studies with large sample sizes and apply inclusion/exclusion criteria ranging from lab results, medication and device use, biometric data, and other critical data points.
To highlight these capabilities, the Truveta team presented new findings on GLP-1s, HIV treatment, and APOE genetic testing. Attendees were particularly interested in diving into usage patterns of newly approved therapies, real-world outcomes associated with specific therapies, and the impact of SDOH factors. In addition to poster sessions, Truveta hosted a theater presentation in collaboration with Dexcom on the SDOH factors impacting type 2 diabetes care and continuous glucose monitor use. The analysis relied heavily on access to clinical notes, as well as SDOH data linked to EHR records – two data types that researchers at the event were eager to gain access to.
Claims usage persists, despite lack of clinical specificity
Claims data continues to be a popular source of real-world data globally, due to its scale and accessibility. In some countries – particularly those with single-payer healthcare systems, claims data can provide longitudinal records of patient interactions with the healthcare system. By contrast, EHR data in these countries may be fragmented and inaccessible due to patient privacy laws and regulations.
However, EHR data offers a better source of longitudinal patient data with rich clinical depth. US patients change insurers every 18 months, making longitudinal data collection via claims incredibly challenging. Additionally, claims data – regardless of country – typically lacks the level of clinical depth and context available within EHR datasets and may suffer from data lags of months to years. For US-based commercial insurers serving specific regions of the country, data may be biased toward the populations represented in those regions, thus not providing a nationally representative view. More importantly, clinical data from EHRs can be used across geographies if treatment patterns are similar, whereas claims data is driven by reimbursement, with costs and healthcare resource utilization differing significantly across countries.
Truveta Data solves for the limitations of claims data by offering full medical records data from a nationally representative population. These records are sourced from more than 30 member health systems that have committed to providing a minimum of five years of longitudinal data upfront, then providing daily updates to further grow the depth of the dataset.
Data quality remains a key priority
Data quality was a key theme at the event, with a focus on how to ensure data is of high quality. Sessions highlighted that data must be trustworthy, relevant, complete, accurate, reliable, and fit for purpose. Attendees were interested in understanding how data quality is measured by RWD providers, whether “missingness” of data is accounted for, and what protocols are used to ensure high data quality.
Truveta’s data quality process extends from the point of ingestion through data normalization and aggregation for research, and is measured along the following dimensions:
- Representativeness: how well patient diversity within a study compares to the overall US population or geography being studied
- Completeness: whether all expected data fields and values are present
- Timeliness: how quickly data is delivered to Truveta and made available for research
- Validity: the degree to which all values are plausible relative to clinical expectations
- Conformance: whether all data elements (e.g., conditions, vitals) have linked fields
Truveta continuously measures more than 500 metrics across all five categories, delivering data with exceptional speed, scale, and depth, while upholding consistent and quantifiable data quality standards.
Integrated datasets save time while adding immense value
Researchers often require a linked dataset containing multiple data types. Third-party tokens and health data marketplaces can, in theory, enable richer datasets, but each linkage may result in decreases in desired patient cohort sizes and the longitudinality and representativeness of the data.
Truveta Data includes deep EHR data linked to claims, social drivers of health, and mortality data, eliminating the need for researchers to manually cobble together these disparate datasets. This level of integration, when coupled with the completeness, representativeness, and longitudinality of the data, enables researchers to gain a much richer and more complete understanding of patient journeys and put findings into context. Access to clinical notes further unlocks insights related to national care delivery patterns and care gaps patients may be experiencing.
We look forward to continued conversations about the potential of EHR data and AI to advance healthcare innovation. Feel free to contact us with questions or to connect.