You probably know someone who will be diagnosed with prostate cancer in their lifetime. 1 in 8 men will develop prostate cancer, with a higher risk in Black and African American men and an overall increased risk after the age of 65. While most cases are not fatal, prostate cancer kills approximately 34,000 Americans every year. Thanks to advances across early detection, imaging, immune and targeted therapeutics, the way we treat prostate cancer is undergoing a radical shift.
For researchers dedicated to advancing treatment for patients with prostate cancer, Truveta provides the most complete, timely, and clean regulatory-grade EHR data for scientifically rigorous research. Truveta Data comes from a growing collective of 30 health systems that provide more than 18% of daily clinical care across the country, representing the full diversity of the United States. Linked with SDOH, mortality, and claims data, Truveta’s daily updated EHR data includes more than 100 million patient journeys, (including lab results and medications) and the largest collection of clinical notes and images integrated with EHR data, including Gleason scores and PET scan findings. Truveta Data also includes patient journeys outside of oncology, such as care provided at urology clinics. This is particularly important for prostate cancer, as early-stage prostate cancer is often treated in urology clinics without the patient seeing an oncologist. Truveta Data includes both the data from urology clinics and the rest of the EHR, including oncology data.
Read on to discover the depth of Truveta Data available for prostate cancer, or explore high-level population details directly in Truveta Studio.
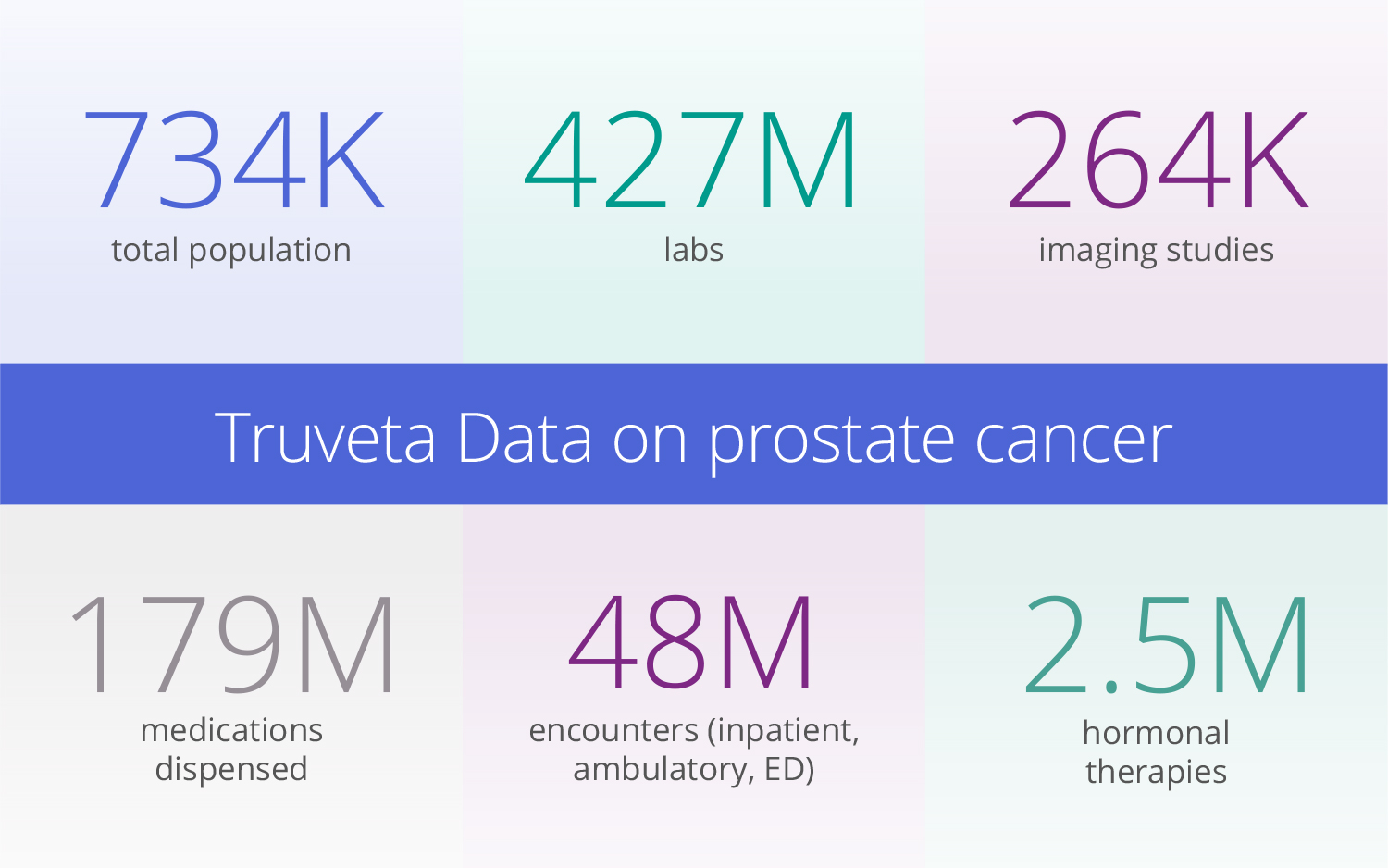
Following the complete prostate cancer patient journey
With Truveta’s regulatory-grade EHR data, prostate cancer researchers can study the entire patient journey with 5+ years of history – including symptom details prior to seeing an oncologist, specific lab results, SDOH factors, and more. Specific measures for the prostate cancer cohort include:
- Prostate-specific antigen (PSA) blood test: 2.4M tests
- Free PSA: 168K tests
- Prostatectomy: 44K procedures
- Sipuleucel-T injection, approved to treat asymptomatic or minimally symptomatic mCRPC (metastatic castration-resistant prostate cancer): 3K medication administrations
- PARP inhibitors, approved to treat mCRPC: 28K medication administrations
- Patients with a prostate cancer diagnosis with household income less than $50K: 169K patients
Between 10-20% of prostate cancer cases will advance to castrate-resistant prostate cancer (CRPC), with most of those CRPC cases diagnosed as metastatic. The mortality prognosis for this stage of disease is poor, emphasizing the importance of monitoring and managing prostate cancer patients to detect progression as early as possible. The tables below represent examples from an mCRPC patient journey to characterize how analysts can examine longitudinal history within Truveta Studio.
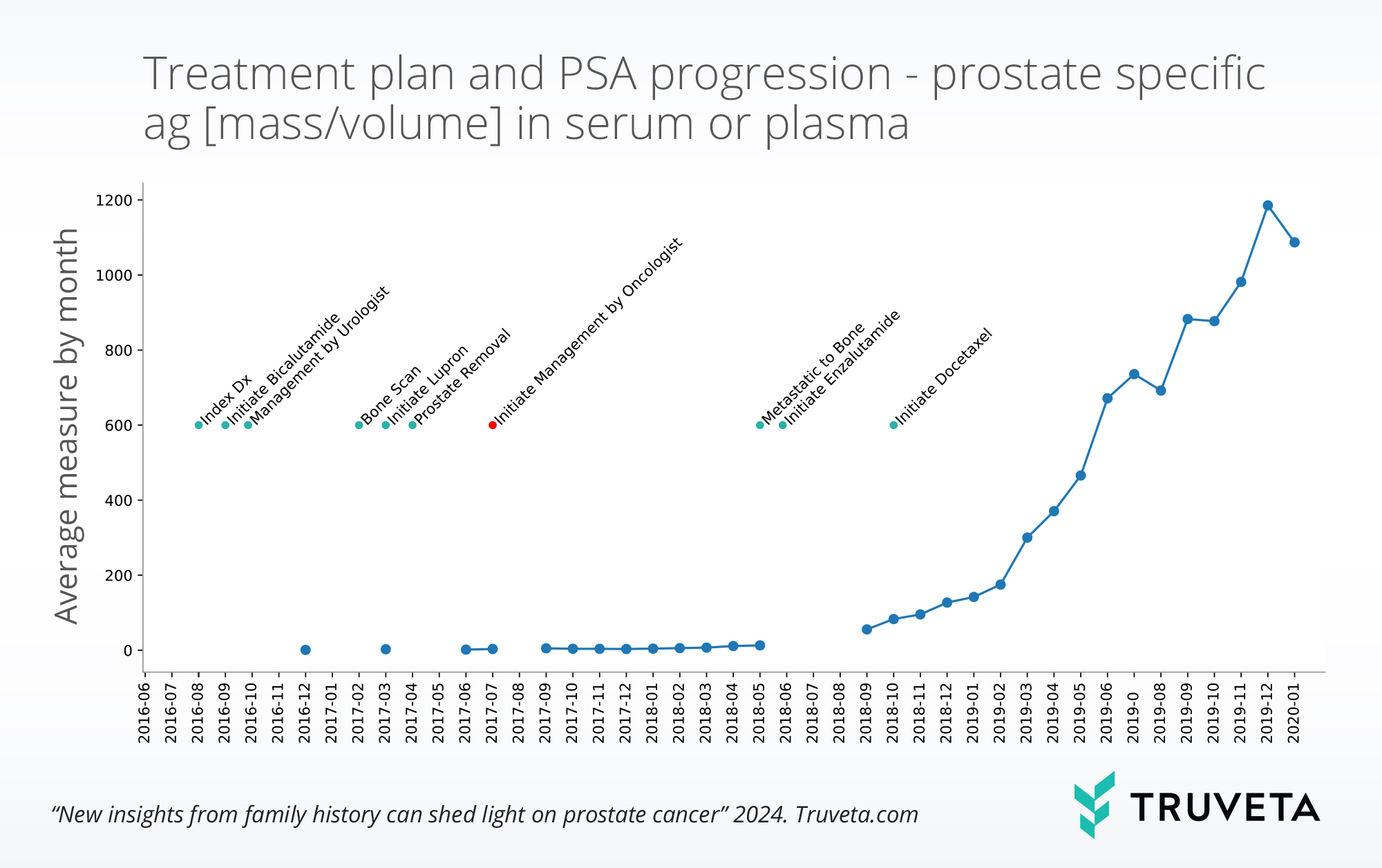
Lab results over time
Lab results and observations can be viewed over time and track values as disease and treatments progress. The graph below shows the extracted average PSA for the sample mCRPC patient by month, with visibility before management by the oncologist.
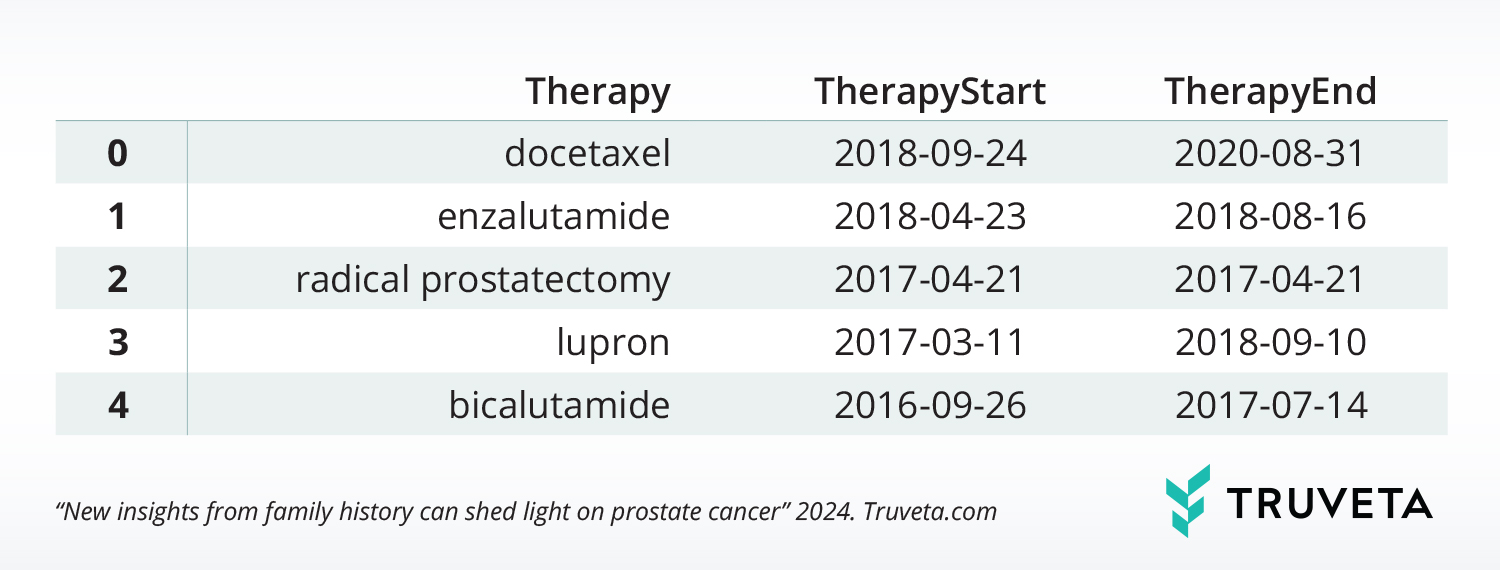
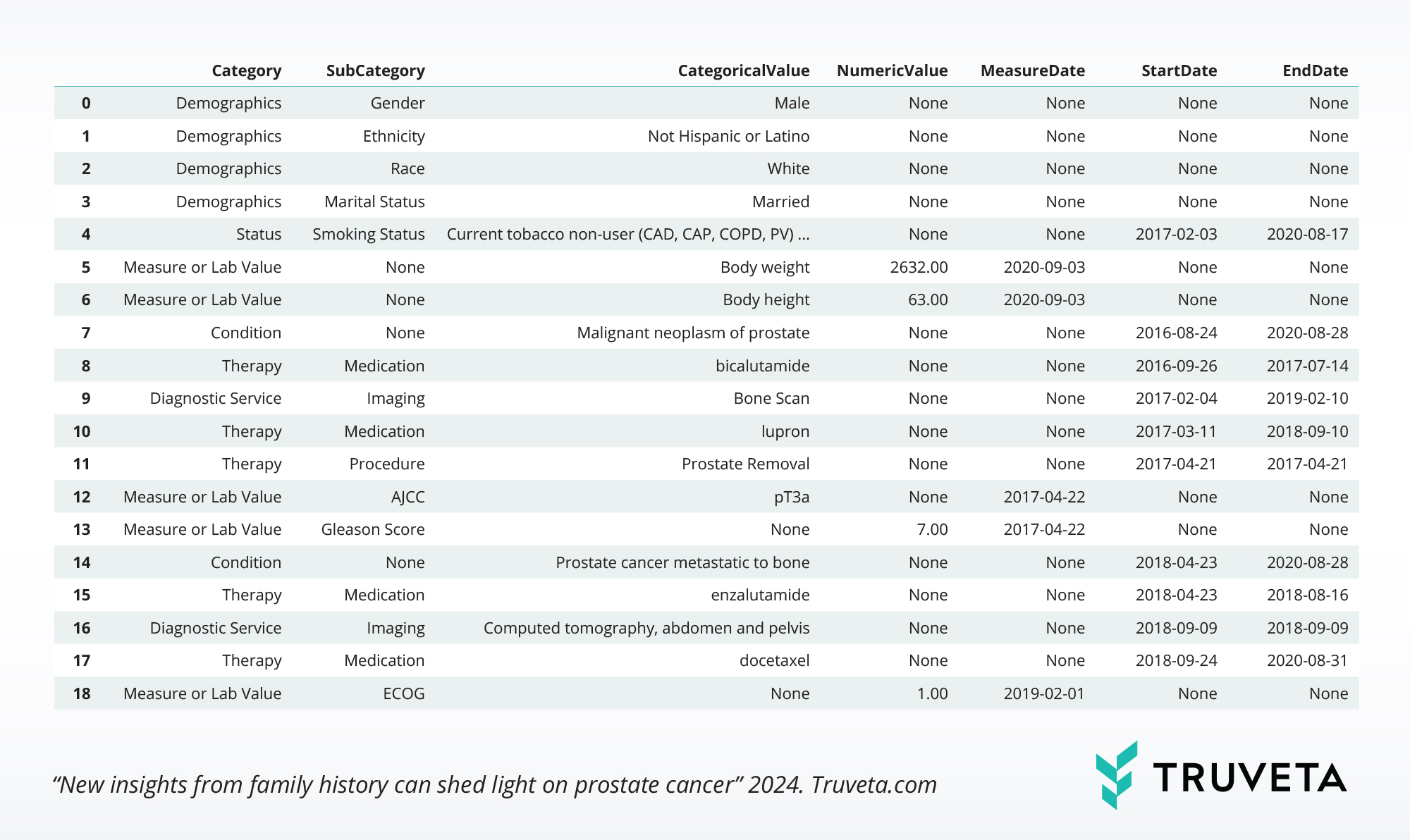
Treatment histories can be constructed from medication administration, medication dispense, medication requests, and claims tables. Below are start and stop dates for a select set of treatments for the sample patient.
More than 5 billion clinical notes available in Truveta Data with clinical expert-led AI
Critical information about a patient’s health – up to 80% of data relevant to research – is hidden in unstructured clinician notes. Truveta uses clinical expert-led AI to extract data from notes at scale, including medication details, family history, frequency and severity of symptoms, and complex concepts from a wide range of clinical areas (ex, Gleason score). The ability to access this data may lead to new insights, such as what can be learned from the 20% of men with prostate cancer who have a family history of the disease.
The sample note below demonstrates examples of highly relevant patient information that can be extracted, structured, and made available to better understand this highly heterogeneous disease.

Researchers can define event tags across all foundational data tables in the Truveta Language Model to construct a study-ready longitudinal view. Additional granularity may include specific lab values, therapy dosage, comorbidities, additional SDOH, and more. The below table includes examples of AI-extracted clinical concepts: surgery staging, Gleason Score, and ECOG score.
Largest collection of medical images integrated with EHR data
Prostate cancer imaging capabilities have advanced within the last several years, with multiple PSMA (prostate-specific membrane antigen) – targeted PET imaging drugs approved by the FDA. These updated tests help physicians to better determine if and where prostate cancer has spread outside the prostate gland. While this data is not routinely captured in real-world datasets, Truveta provides researchers with the largest and most complete medical imaging dataset to more deeply understand symptoms, diagnoses, disease progression, and more. These PET scans – as well as other imaging modalities including MRI, CT, X-ray, ultrasound, and nuclear medicine – are integrated with EHR data.
The Truveta Data prostate cancer population includes 264K imaging studies, and researchers can use the new imaging viewer in Truveta Studio to view thousands at a time. These de-identified images can also be analyzed in notebooks in Truveta Studio and exported for studying imaging-based outcomes and observations, AI/machine learning model development, or inclusion in publications.
The next era of prostate cancer treatment
We are entering an era where management and mitigation of prostate cancer can be as precise as it is proactive. By providing the most complete view of the patient journey, Truveta empowers researchers to accelerate the adoption of new therapies, improve clinical trials, and enhance patient care for the millions of men living with prostate cancer.
Learn more and request a demo: Contact us