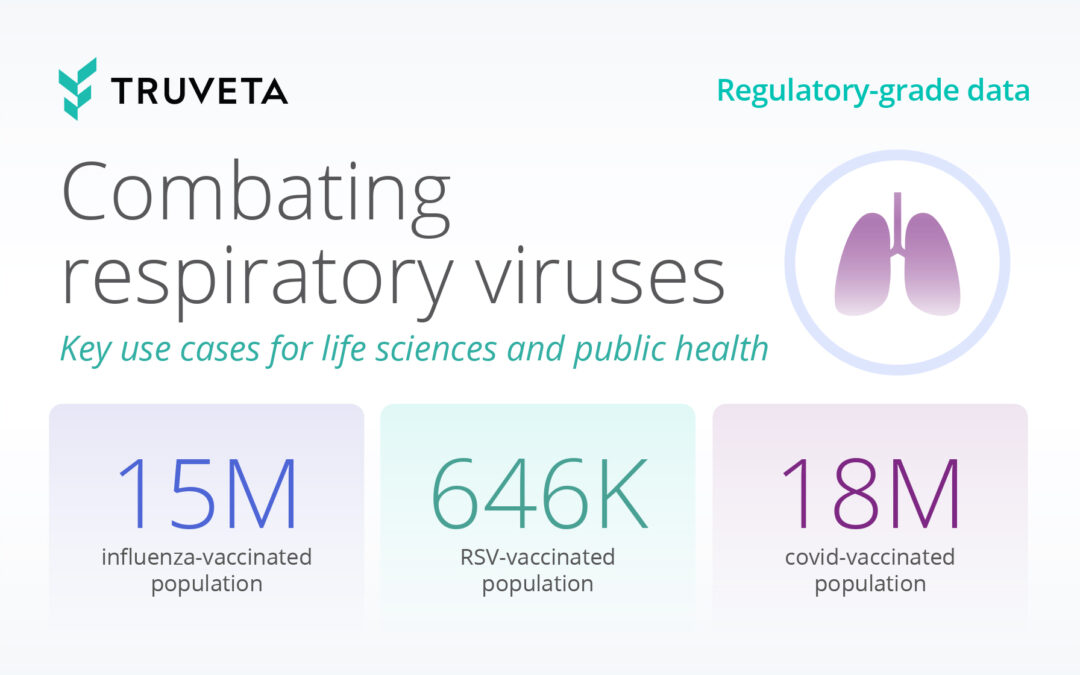
Why autoimmune research needs better real-world data
Biologics like Humira, Enbrel, and Rinvoq have transformed autoimmune treatment, revolutionizing treatment for patients with chronic inflammatory infections. However, the traditional methods of studying these therapies—randomized controlled trials (RCTs) and clinical registries—are too slow, too expensive, and often lack real-world generalizability.
As the autoimmune landscape evolves with new therapies and increasing biosimilar competition, life sciences leaders require fast, comprehensive insights to track treatment performance across diverse patient populations. Truveta Data delivers a real-world, regulatory-grade alternative, replacing or complementing outdated registries and clinical trials with real-world evidence (RWE).
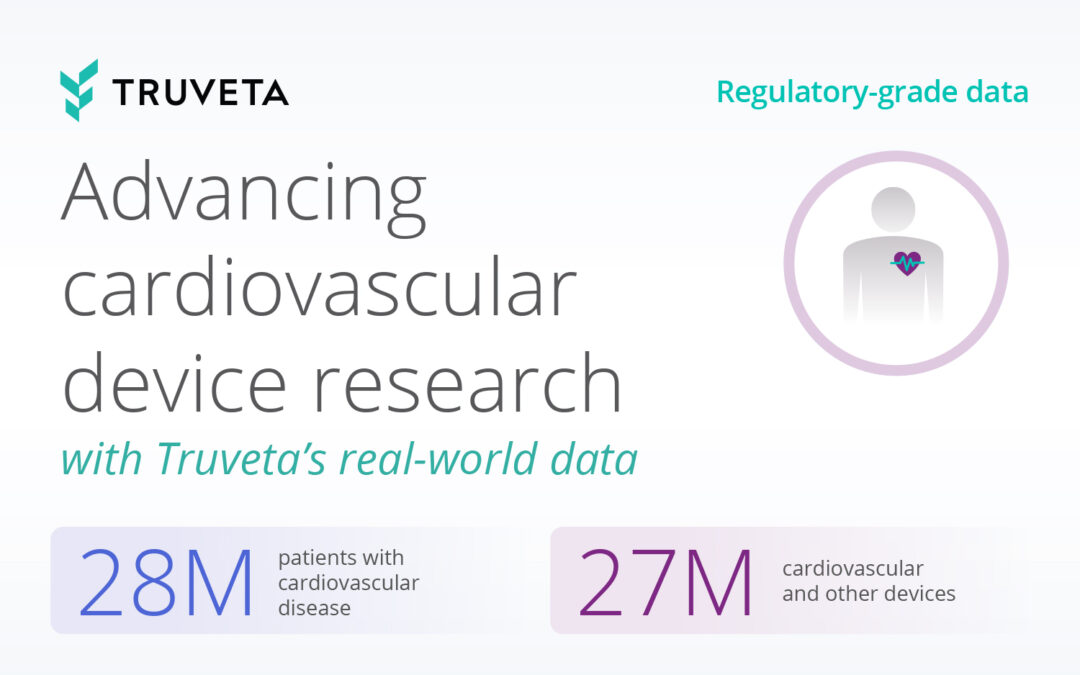
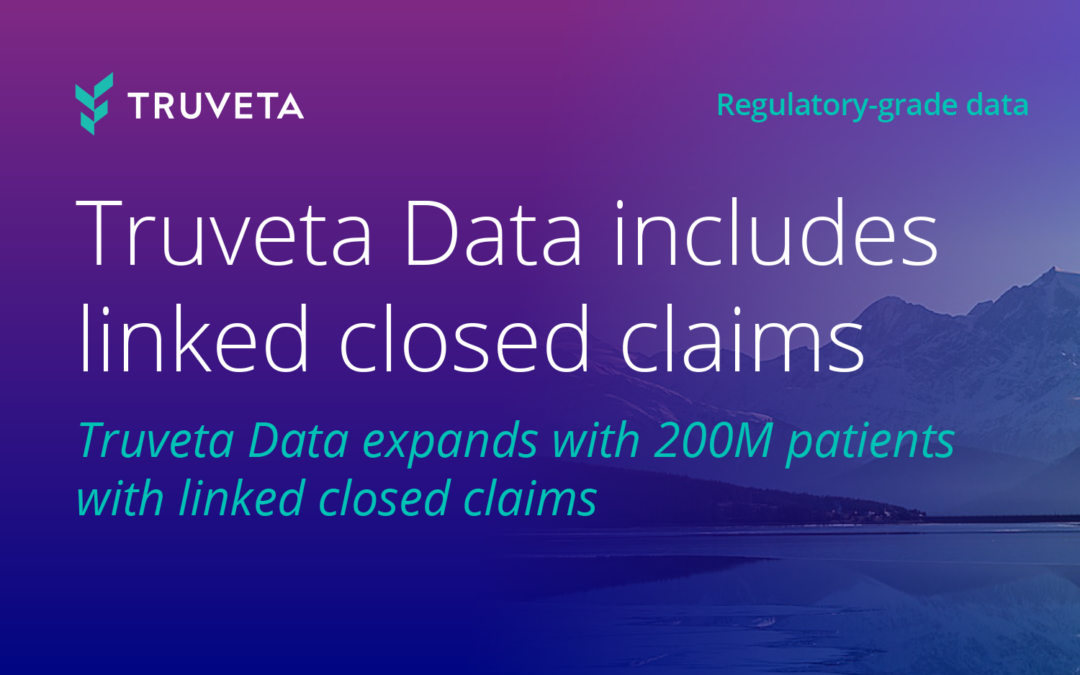
Truveta’s regulatory-grade data provides electronic health records from 120 million patients along with closed claims from 200 million patients, providing the most representative, complete, and timely patient journey data – exceeding FDA standards. With this depth, researchers can monitor treatment switching, adherence, safety outcomes, and health disparities in real-time.
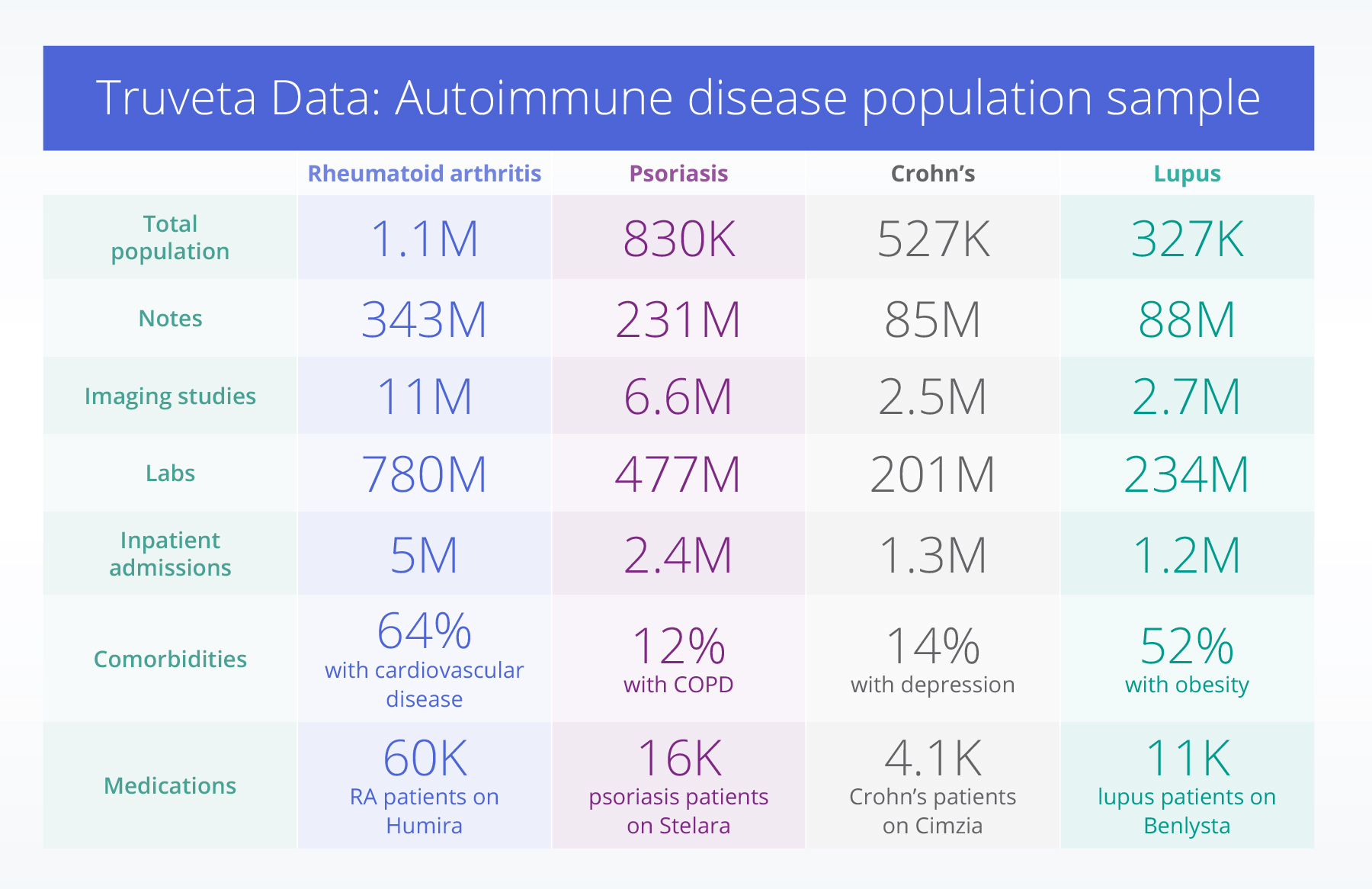
The power of real-world data in autoimmune research
Understanding how pharmaceutical treatments are used in autoimmune diseases and their impact on long-term patient outcomes requires longitudinal data, which traditional trials struggle to provide. Treatment decisions, ranging from first-line NSAIDs and DMARDs to biologics and JAK inhibitors, are shaped by factors like clinical effectiveness, payer coverage, and social drivers of health (SDOH).
Truveta enables researchers to:
- Track switching behavior between biologics and biosimilars in response to policy changes
- Analyze real-world adherence trends based on demographic and socioeconomic factors
- Evaluate safety signals post-market, reducing reliance on costly, long-term safety trials
Real-world case study: Biosimilar adoption and market forces
Truveta Research recently conducted a study on medication switching trends from Humira to approved biosimilars following the CVS formulary change that removed Humira in favor of its biosimilar, Hyrimoz. Biosimilar switching was uncommon during 2023 but increased significantly in 2024. Truveta Data showed that 86.9% of all switches to biosimilars occurred in January 2024 or later, aligning with the formulary update.
Key takeaways:
- Switching increased more than tenfold after CVS made the change
- 77% of patients who switched moved to the CVS-preferred biosimilar (Hyrimoz), rather than other available options
- Switching to interchangeable versions of the drug remained low, suggesting that cost and insurance coverage may play an important role in switching
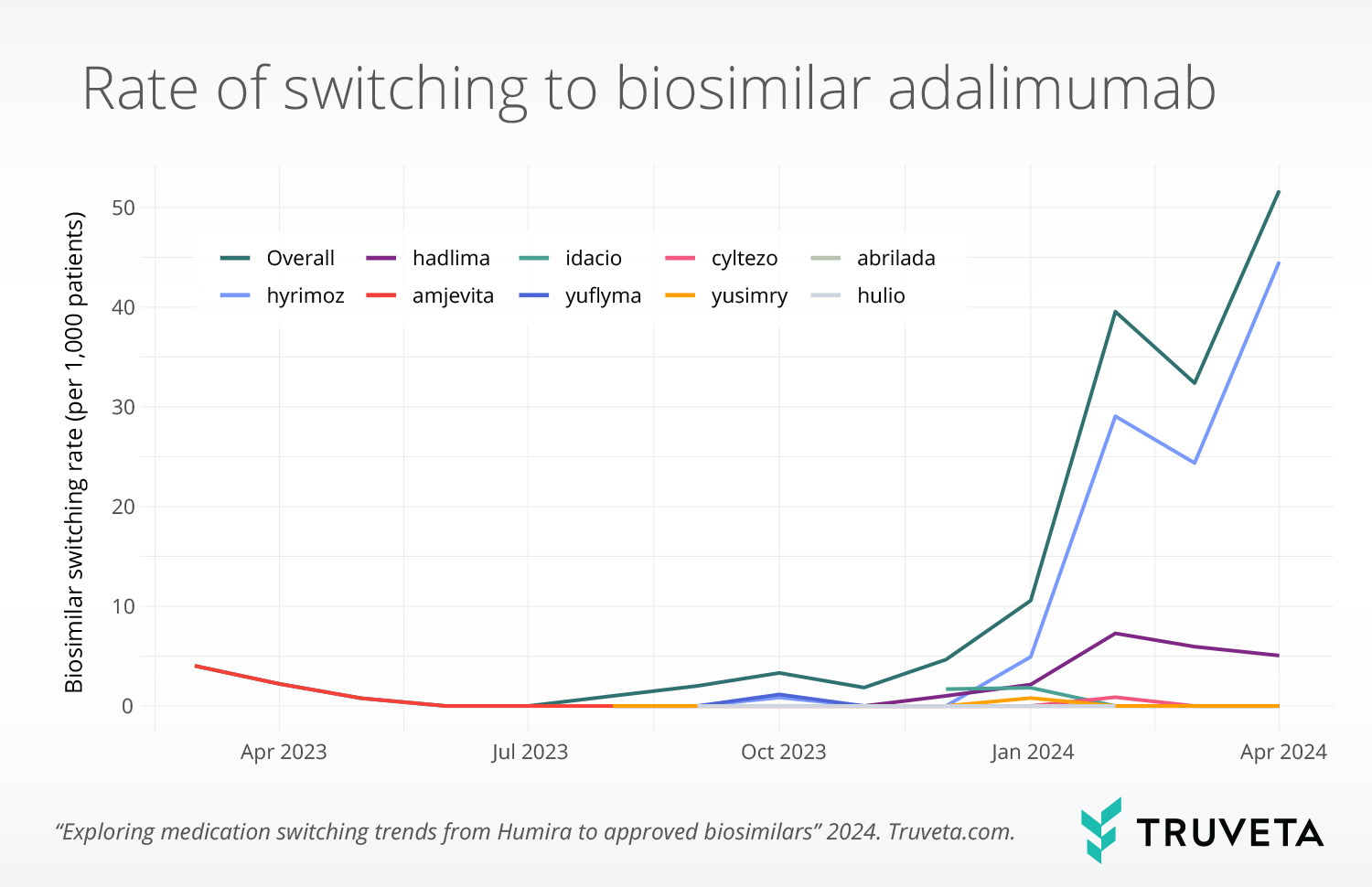
This study highlights how insurance policies—not just clinical evidence—drive treatment adoption. For pharmaceutical companies, this underscores how real-world data is key to tracking how coverage changes impact prescribing behavior and biosimilar adoption.
Largest collection of clinical notes and images
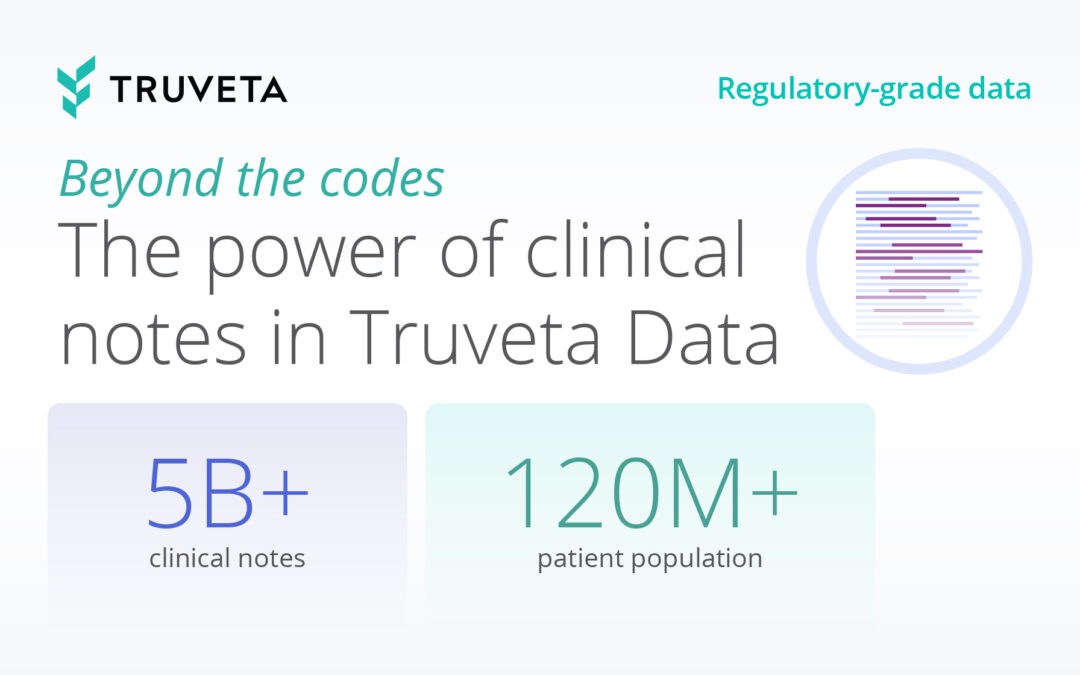
One of Truveta’s biggest differentiators is the inclusion of over 5 billion clinical notes and more than 100 million medical images from all settings of care, making it the largest collection of notes and images integrated with EHR data available for autoimmune disease research.
Researchers can go beyond structured EHR data to analyze 11 million imaging studies for rheumatoid arthritis, 6.6 million for psoriasis, 2.5 million for Crohn’s, and 2.7 million for lupus, monitoring disease progression, treatment impact, and long-term patient outcomes. For example, these images provide visual evidence of disease severity, such as joint damage in rheumatoid arthritis, bowel inflammation in Crohn’s disease, or skin lesion progression in psoriasis.
With access to all clinical notes generated during a patient’s care, researchers can also analyze physician observations, symptom descriptions, and treatment rationales. Truveta’s AI-powered Truveta Language Model extracts clinical concepts from notes at scale, linking them to structured data to enable robust research across therapeutic areas.
A clinical note for a patient with psoriasis may describe the frequency and severity of flare-ups, treatments attempted, and impact on quality of life. Now, researchers can systematically study these details across millions of patient records, unlocking new insights into how autoimmune diseases manifest, progress, and respond to treatment over time.

This level of clinical depth is invaluable not only for understanding patient journeys across immunology and other disease areas, but for accelerating therapy development and adoption, optimizing treatment guidelines, and supporting regulatory submissions.
The future of autoimmune research with Truveta
The next frontier for Truveta is the integration of genomics data through the Truveta Genome Project. Many autoimmune diseases remain poorly understood at the genetic level, and genomics could be the key to identifying disease origins, predicting likelihood of diagnosis, and personalizing treatment pathways. By combining genomic insights with real-world clinical data, researchers will soon be able to uncover new therapeutic targets, enhance precision medicine approaches, and develop more effective treatment strategies for autoimmune diseases.
As for today, real-world data in autoimmune research is already shaping regulatory decisions, with the FDA and CMS actively incorporating real-world evidence (RWE) into their review processes. Truveta provides a scalable, regulatory-grade replacement to traditional clinical trials and registries, enabling life sciences companies to:
- Conduct real-time monitoring of therapy adoption and adherence
- Use real-world effectiveness data for label expansion and payer negotiations
- Enhance safety surveillance to predict and mitigate adverse events
As autoimmune diseases continue to rise—with prevalence increasing by an estimated 3-12% annually—real-time insights will be essential. Truveta offers the most representative, complete, and timely data available to help drive innovation and improve patient outcomes.