Mother and child health are inherently interconnected, offering unique insights into how maternal health, behaviors, and exposures impact child outcomes. Unfortunately, the potential of this data has historically been limited by challenges in data collection, the long timeframes required for follow-up, and the complexities of linking records across disparate systems. Today, the availability of electronic health records and advanced data-linking technologies offer researchers unprecedented access to real-time, longitudinal data. Truveta’s daily-updated EHR data, encompassing more than 1 million directly linked mother-child pairs, represents a transformative resource for medical research.

Why research on mother-child data is important
Understanding the link between maternal health and child outcomes is crucial for advancing both maternal and pediatric care. Research in this area can lead to significant discoveries, such as identifying risks associated with specific medications during pregnancy, the role of maternal behaviors on child health, and the mechanisms of mother-to-child transmission of infectious diseases.
Improving maternal & infant care with Truveta’s real-world data
Now, with the largest mother-child EHR dataset, researchers can better understand maternal and infant health patterns and explore trade-offs at scale. Truveta enables researchers to conduct safety monitoring and comparative effectiveness research to improve patient care, with access to longitudinal clinical data that follows mothers and their children throughout the entire pregnancy journey and up to 5 years after birth. Read on for the top 10 reasons to choose Truveta’s mother-child dataset.
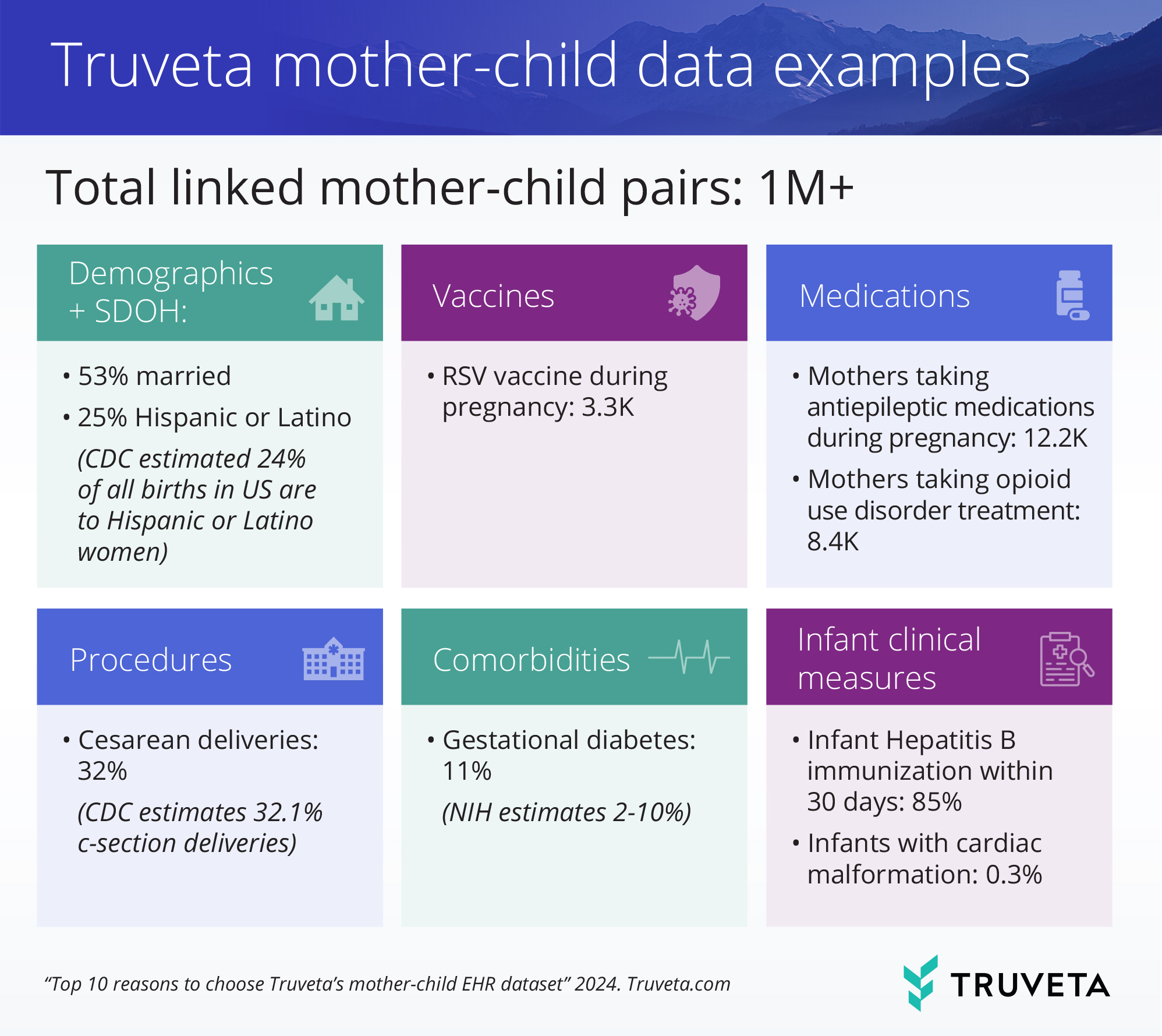
1. Comprehensive and representative coverage
Truveta offers the most complete EHR dataset, with data sourced directly from a growing collective of member health systems representing the full diversity of the United States. These records preserve critical elements such as demographics, SDOH indicators, medical history (including all medications and immunizations), pregnancy outcomes, diagnoses, procedures, and more. For children, detailed delivery data is available, including type of delivery, complications, gestational age, Apgar scores, and lab results at time of birth, as well as all diagnoses, procedures, and immunizations up to age 5.
2. Accurate and direct linkage of mother-child pairs
Truveta Data establishes a direct linkage between mother and child using birth events in the EHR, ensuring accurate, explicit, and reliable data obtained directly from patient records. Unlike claims-based datasets that may suffer from false matches or commercial bias (excluding patients on Medicaid or the uninsured), Truveta provides a more precise and representative linkage of mother-child pairs.
3. Access to clinical notes for deeper insights
Truveta integrates the largest collection of clinical notes with EHR data, empowering researchers with previously hidden information. This includes progress notes, procedure and operative reports, referral notes, discharge summaries, and imaging reports. The Truveta Language Model extracts data from these notes at scale, allowing systemic analysis of any clinical concept of interest.

4. Incorporation of medical imaging data
Medical images are integrated with EHR data, providing a complete picture for research across modalities and therapeutic areas. De-identified images, along with pixel data and imaging metadata, can be previewed and analyzed within Truveta Studio. Images can also be exported for studies on imaging-based outcomes and observations or for inclusion in publications, enhancing the quality and scope of the research.

5. Deep understanding of maternal health impact on child outcomes
Truveta’s mother-child dataset empowers researchers to explore critical connections between maternal health and neonatal outcomes, including preterm delivery, neurodevelopmental outcomes, and various childhood conditions up to age 5. For example, the dataset includes 0.3% of linked children with cardiac malformation, allowing for focused studies on specific health outcomes.
6. Monitor post-market safety for medications taken during pregnancy
The dataset allows for robust monitoring of post-market safety data for chronic disease medications administered during pregnancy, critical for regulatory submissions. Researchers can track outcomes such as conditions, hospitalizations, and mortality rates in children born to mothers who took specific medications, such as anti-seizure drugs, providing valuable insights for regulatory bodies and healthcare providers.
7. Evaluate vaccine safety and uptake among pregnant women
With all immunizations received during pregnancy available for research, Truveta supports critical studies on vaccine safety and effectiveness. This is particularly important given that vaccines for influenza, Tdap, RSV, and COVID-19 are recommended during pregnancy. Truveta Data also enables examination of racial disparities in vaccination rates among pregnant women.
8. Investigate the effects of Category C medications during pregnancy
Truveta enables the study of Category C medications and their effects on pregnancy and health. These medications, which have shown risks in animal studies but lack well-controlled human studies, pose complex decisions. Pregnant women must consider whether the potential benefits of the drug outweigh the risks, often with little information. Researchers can now analyze how these drugs impact children, especially given that a recent review of 103 relevant drugs found that 77% had inconsistent recommendations for pregnant women.
9. Study the correlation of pediatric conditions with SDOH and maternal factors
Truveta’s mother-child dataset provides a unique opportunity to explore correlations between pediatric conditions such as asthma or eczema, linked SDOH factors (like marital status, education, and access to transportation), medical history, and pregnancy outcomes. This facilitates more comprehensive research on the interplay between health outcomes and socio-economic factors.
10. Regulatory-grade privacy and security compliance
Protecting the privacy and security of this important data is critical. Truveta is committed to the highest standards of privacy and security. Truveta’s security has earned SOC 2 attestation and multiple ISO certifications. Also, external experts validate Truveta’s privacy program to ensure it meets stringent HIPAA requirements and review its de-identification process, giving researchers confidence in the safety and integrity of the data they are using.
Interested in learning more about Truveta Data?
Request a demo with our team: Contact us
