Truveta now offers regulatory and audit capabilities to support our customers for real-world evidence (RWE) submissions to the Food and Drug Administration (FDA) and other global regulatory authority decisions. These regulatory grade capabilities advance Truveta’s safety, health economics and outcomes research (HEOR), and clinical trial solutions for life sciences.
Real-world evidence is the clinical evidence regarding the usage, and benefits and risks, of a medical product derived from the analysis of real-world data (RWD). The FDA has relied on RWE to support regulatory decisions to help speed patient access to innovations that advance public health, noting that RWE can be leveraged to bring new products to market, evaluate the safety and effectiveness of existing products for new uses, and assess the continued performance and safety of products once on the market.
The FDA suggests the real-life clinical performance of a medical product may be more clearly demonstrated through RWE and RWD because a controlled clinical trial often cannot evaluate all applications of a product in clinical practice across the full range of potential users.
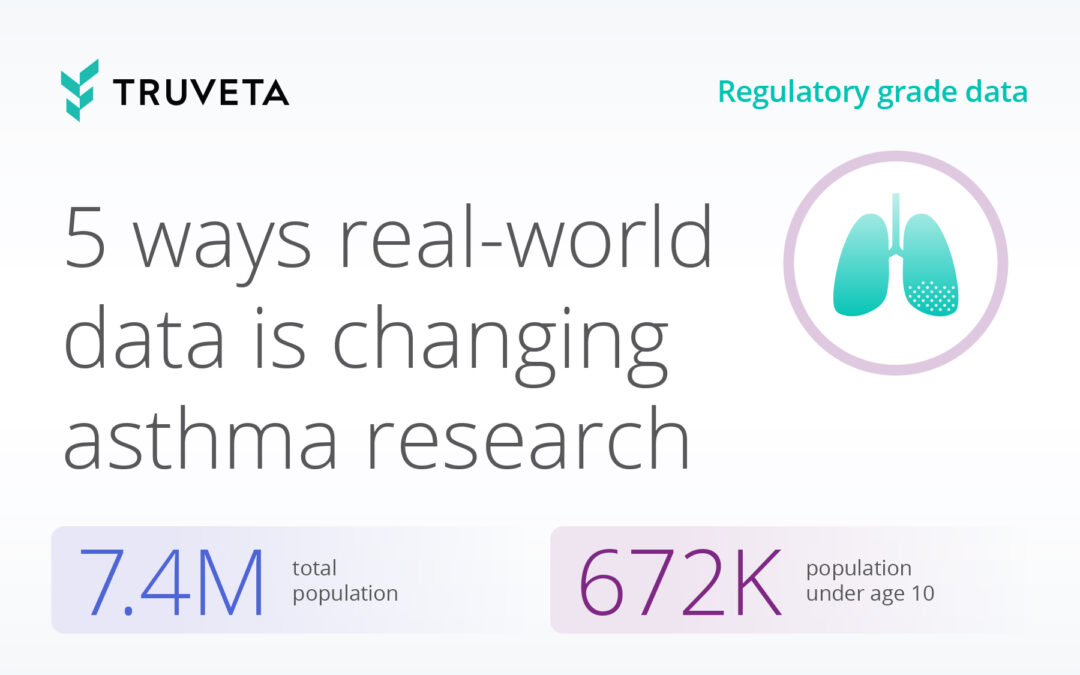
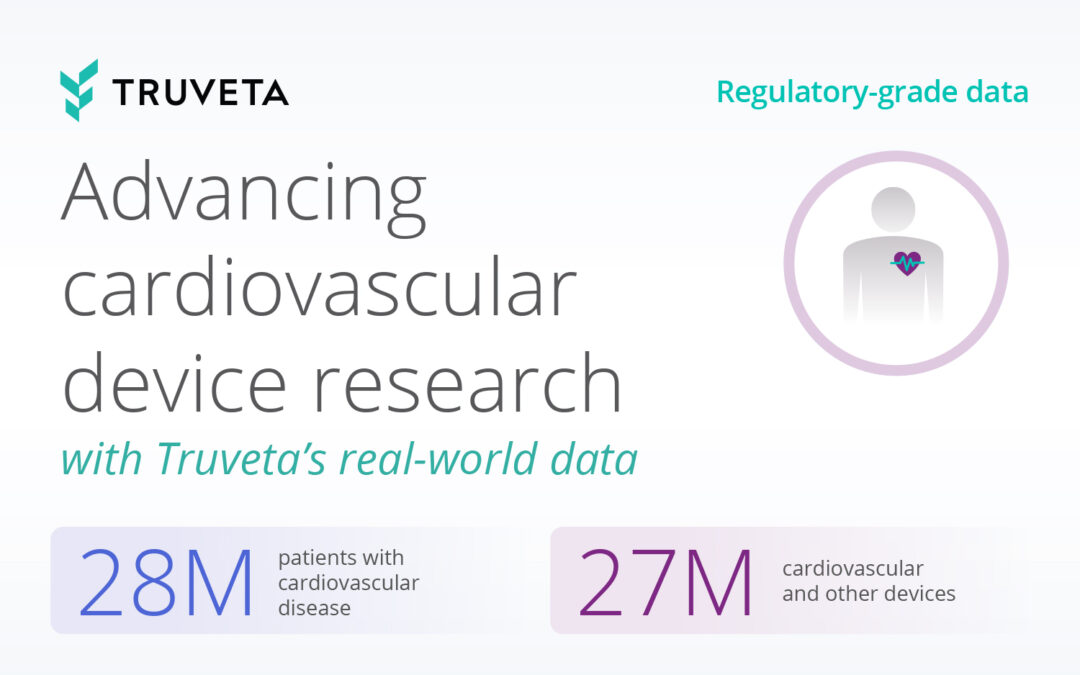
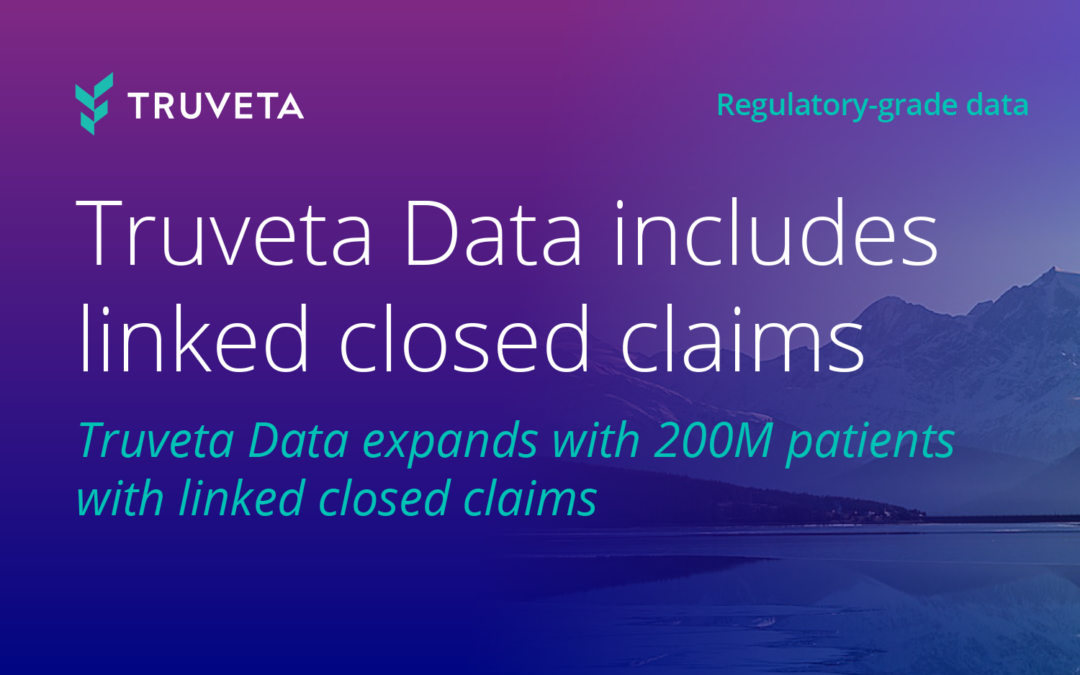
Truveta offers the most complete, timely, and clean regulatory-grade electronic health record (EHR) data from more than 100 million patients across 30 US health systems, empowering researchers to study all diseases, drugs, and devices. Truveta Data is representative of inpatient and outpatient care from over 900 hospitals and 20,000 clinics. Truveta Data is updated daily for the most current view of patient care. By providing a complete view of the patient journey, including clinical notes and medical images, Truveta enables researchers to accelerate therapy approval and adoption, and enhance patient care.
Aligned with guidance published by the FDA, we have invested deeply in establishing rigorous and demonstrable standards of data quality and provenance, workflow support for regulatory submissions, and audit-ready processes, procedures, and controls to support life sciences organizations in meeting the most stringent requirements of major regulatory bodies.
Truveta Data endorsed as research-ready for regulatory submissions
Key areas of investment into Truveta Data include a state-of-the-art data quality management system (QMS), third-party system audits by regulatory experts, and industry-leading security and privacy certifications.
- Truveta’s quality management system ensures data integrity. In accordance with regulatory guidance, we have implemented a robust quality management system to ensure the highest standards of data integrity and continuous improvement. Data quality metrics include representativeness, completeness, cleanliness, and timeliness. Each of these must be met from the point of ingestion through multiple stages in the data pipeline. Truveta has full transparency into the provenance of each data point given our unique relationship with health system members, who provide the data for Truveta Data.
We have designed hundreds of product and process improvements to exceed FDA regulatory expectations, implemented more than 70 process and procedure controls aligned with FDA guidance, and created more than 30 standard operating procedures focused on regulatory requirements. Each component within Truveta has a standard operating procedure in which risks are identified and purpose-built processes and software controls are written. Continuous monitoring and evidence logging ensure the system complies with these standards and that Truveta customers can prove the integrity of the data and documentation included in their regulatory submission.
- Truveta Language Model is accurate, transparent, and accountable. Truveta Language Model (TLM) is a large-language AI model used to clean billions of daily EHR data for scientifically rigorous research. TLM’s healthcare expertise is trained on Truveta Data, the largest collection of complete medical records representing the full diversity of the United States. TLM cleans concepts from unstructured clinical notes. Each clinical concept extracted from notes is accompanied by documentation of the concept definition, modeling methods, and the precision/recall of TLM’s extraction. This documentation is designed to be shared as part of regulatory-grade evidence submission. You can learn more in the Truveta Language Model whitepaper.
- Truveta Data is audit ready. All data and documentation are stored for the duration required by the FDA and are accessible by the researcher if or when an audit occurs. With secure storage and versioning of all data, metadata, analytic code, and evidence, researchers can readily retrieve evidence for regulatory audits upon request. Logs proving system integrity during artifact creation with direct linkages to the artifacts themselves are stored and can also be shared.
- Type 2 SOC 2 attestation and ISO 27001, 27701, 27018 certifications demonstrate Truveta’s relentless commitment to security and privacy. To protect Truveta Data, we developed one of the most advanced data security and privacy systems in the healthcare industry. Truveta’s systems protect data through every stage of this process and have been validated to meet the most rigorous standards for privacy and security. Truveta has earned Type 2 SOC 2 examination and ISO 27001 certification, with ISO 27018 and 27701 extensions, with additional certifications underway. These certifications serve as external validation that Truveta’s controls, protocols and processes align with rigorous standards for both security and privacy.

New regulatory submission capabilities in Truveta Studio
In addition to investments in Truveta Data, we have also delivered new advancements to Truveta Studio, our analytics platform, to support customers at each stage of the regulatory process, including artifact creation, regulatory submission, and any regulatory audits.
Customers can:
- Classify a study as intended for regulatory submission upon creation. This makes the study identifiable as a regulatory artifact and unlocks features for evidence generation, regulatory exports, and default data retention policies.
- Classify a population snapshot as intended for regulatory submission. This defaults the snapshot to an indefinite retention policy and administrator privileges are required for deletion. Additional evidence is generated to link the snapshot, to be used as source data for a submission, to the system controls validating its integrity at the point of generation.
- Classify a notebook analysis as intended for regulatory submission upon finalization. This also generates additional evidence linking the finalized analysis to the controls demonstrating its integrating.
- All regulatory artifacts can be placed into a read-only and verifiable export supporting regulatory submission.
- All logged evidence proving the integrity of system processes involved in artifact creation is stored internally and readily available. It can be provided easily to an auditor upon request, ensuring that Truveta customers are well supported during a regulatory audit.
New regulatory professional services available from Truveta
In addition to embracing a growing partner ecosystem, we now also provide regulatory professional services, including support for post-marketing commitments and/or requirements (PMC/PMR) and post-authorization safety and/or efficacy studies (PASS/PAES). Organizations can use Truveta to monitor safety and effectiveness, especially in populations not typically represented in a clinical trial setting, as well as evaluate real-world clinical effectiveness, adherence, and dosing.
Truveta also now supports label expansion efforts, such as understanding safety and effectiveness in broader populations compared to the approved label, evaluating how real-world dosage changes may impact safety and efficacy compared to the approved dosage, and exploring comparative effectiveness and safety against standard of care or other comparators.
Supporting these services is the Truveta Partner and dedicated Regulatory Success team, whose collective experience and credentials include over 170 publications, nearly 11,000 citations, over 200 conference presentations and posters, and dozens of regulatory projects across numerous state, federal, and international governments and agencies.