- Lecanemab received accelerated FDA approval in January 2023 and full FDA approval in July 2023. A potential side effect of the drug is brain swelling and/or small hemorrhages and is most common for people with the APOE ε4 genetic variant, therefore rates of APOE e4 genetic testing among people with Alzheimer’s or mild cognitive impairment may be an early indicator of intention to use lecanemab. We wanted to expand our previous analysis to understand social drivers of health (SDOH) for people receiving APOE genetic testing and lecanemab.
- We found higher incomes for both the population receiving APOE genetic testing and lecanemab compared to the overall population. The group who received APOE genetic testing was younger than the overall population and the group with lecanemab was younger than both groups.
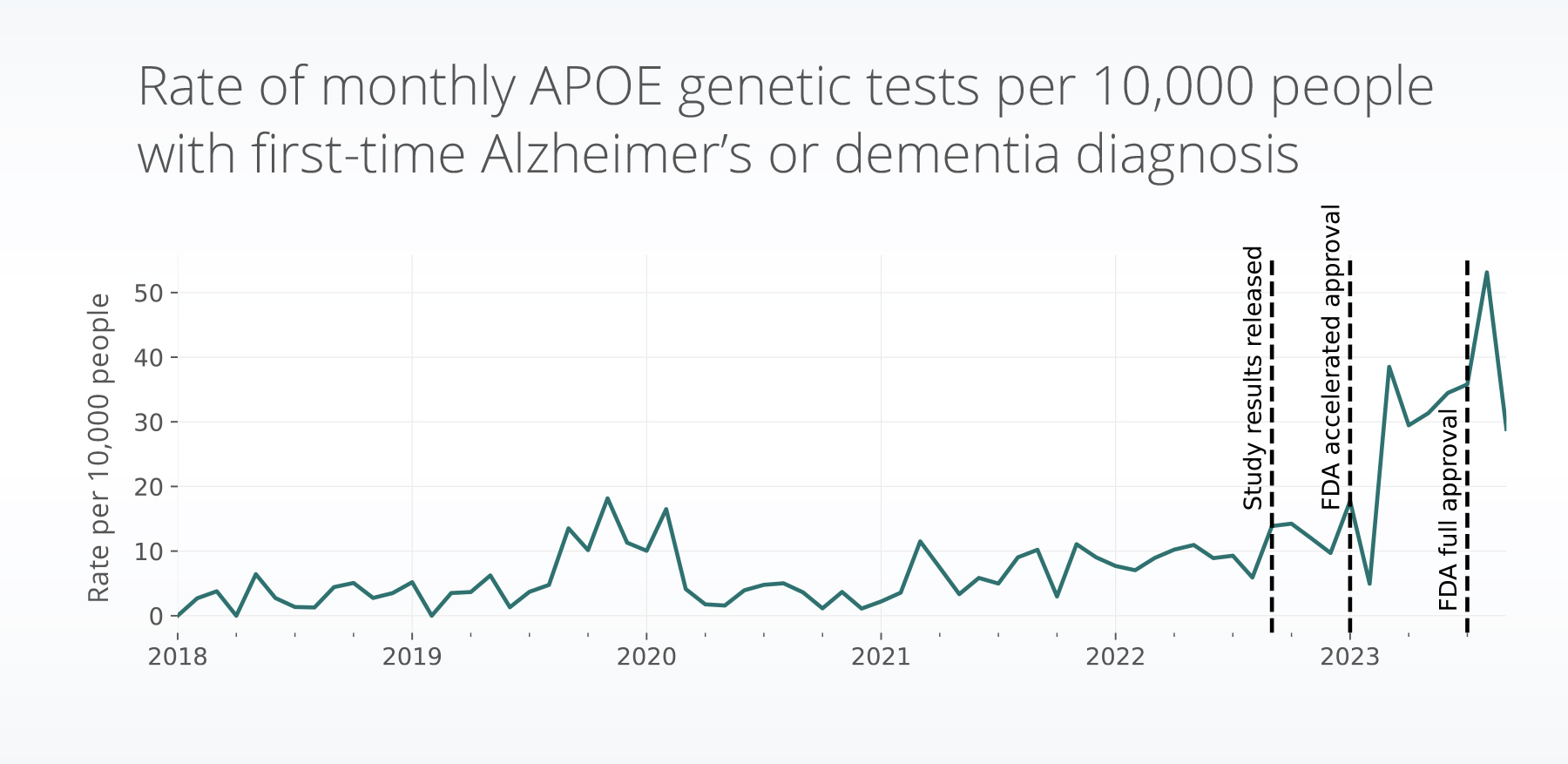
- We also found a significant increase in rate of APOE genetic testing since January 2023. Between April – September 2023, we saw a 3.6x increase in the rate of tests, compared to one year prior.
This blog is an extension of our poster presented at ISPOR Europe 2023, titled Real-World Trends in APOE Genetic Testing Associated with Lecanemab.
APOE genetic testing is recommended for people seeking treatment for early onset Alzheimer’s Disease or dementia with lecanemab (Leqembi) (Eisai Global, 2023). Clinical trial results showed people who have the APOE ε4 genetic variant experienced a higher incidence of brain swelling and/or small hemorrhages, collectively known as amyloid-related imaging abnormalities (ARIA), than non-carriers (van Dyck et al., 2023).
We have previously shared data showing an increase in APOE genetic testing through January 31, 2023. However, since we last shared data, lecanemab has received full FDA approval (US Food & Drug Administration, 2023), leading to more widespread insurance coverage for lecenemab (by Medicare). Not only were we curious to learn who are the people receiving lecanemab, but with its high cost, we were also curious to investigate differences in social drivers of health (SDOH) values for people receiving APOE genetic tests and lecanemab.
Methods
Using a subset of Truveta Data, we identified people with a first-time Alzheimer’s or dementia diagnosis between January 1, 2016, and September 30, 2023. Within this population we identified people who received APOE genetic testing (defined by a lab test) and people who received lecanemab (either through a prescription, administration, or claim).
Population demographics and SDOH
We describe the demographics and social drivers of health (SDOH) for the population with an Alzheimer’s or dementia diagnosis. We investigated five SDOH factors: 1) own or rent current place of living, 2) distance to closest first-degree relative, 3) college attendance, 4) address stability (measured by the number of moves within the last 12 months), and 5) individual income.
APOE testing trends
We plot the monthly rate of APOE tests per patients with a first-time Alzheimer’s or dementia diagnosis. We used an autoregressive integrated moving average (ARIMA) model to test for changes in the rate overtime and describe the outputs.
Lecanemab trends
We describe demographic and SDOH characteristics for patients who were prescribed or administered lecanemab and received an APOE genetic test. We also describe the time between first-time diagnosis, APOE genetic testing, and subsequent lecanemab prescription/administration. For the purposes of this study, although the prescription and administration date may be different, the first date was included if both were available.
Results
We included nearly 800,000 people with an Alzheimer’s or dementia diagnosis. Within that group, 578 people received an APOE genetic test, and 44 people received a prescription, administration, or claim for lecanemab.
Population and SDOH
The population with Alzheimer’s or dementia was primarily female (60.0%) and 75 years of age or older at the time of their first diagnosis (67.5%). The population who received APOE genetic testing owned their current place of living at a higher rate (81.0% of those with APOE genetic testing, compared to 75.5% in those without APOE genetic testing) and a higher proportion (95.0%, compared to 92.8%) lived within 25 miles of their closest first-degree relative. A higher proportion of the group that received APOE testing had incomes over $100,001 (21%), compared to the group that did not receive testing (4.1%).

APOE testing trends
The rate of APOE genetic testing has significantly increased since January 2023 (accelerated approval granted, p = 0.02) and continues to increase in trend (p < 0.001). Between April – September 2023, we saw a 3.6x increase in the rate of tests, compared to one year prior.
Lecanemab trends
Within our population, we saw evidence of 44 people receiving lecanemab. Compared to the overall Alzheimer’s or dementia population, a larger percentage of the population that has received lecanemab was male (52.3%) and younger (63.6% of the population was under 75 years old). Most of the population was white (97.7%), owned their place of living (92.7%) and had higher incomes than the overall population. The first prescription/administration occurred 63.3±53.9 days after the APOE genetic test and 519.0±494.4 days after the initial diagnosis.
Discussion
Lecanemab is a new drug that has inspired hope for a people with a disease that has not had effective treatments. However, some people may be at additional risks of brain swelling and/or small hemorrhages due to a genetic variant in the APOE gene. Genetic testing for APOE has been recommended for people starting lecanemab.
Since both the FDA accelerated approval (in January 2023) and full FDA approval (in July 2023) of lecanemab (which led to increased insurance coverage for lecanemab) we have continued to see increases of APOE genetic testing. Additionally, the population receiving both testing and lecanemab have a higher socioeconomic status. With the high cost of lecanemab, this may indicate disparities in the patient populations who able to access the drug and potentially receive its benefits.
We still see only a small sample receiving lecanemab; therefore, we will continue to monitor both uptake in lecanemab and associated APOE genetic testing.
There are a few limitations with this analysis; first we are not capturing APOE tests that do not occur within the hospital system. As these tests are relatively inexpensive (USD$125 for a one-time test), it is possible that people are receiving tests outside the hospital system. Further, we included prescriptions, administrations, or claims to pay for lecanemab. When comparing differences in time between diagnosis or prescription/administration/claim, the time may be different; however, we included one as the time point in this analysis.
These are preliminary research findings and not peer reviewed. Data are constantly changing and updating. These findings are consistent with data accessed on October 16, 2023.
Citations
Eisai Global. (2023, March 6). FDA Accepts Eisai’s Filing of a Supplemental Biologics License Application and Grants Priority Review for Traditional Approval of LEQEMBITM (lecanemab-irmb) for the Treatment of Alzheimer’s Disease. https://www.eisai.com/news/2023/news202316.html
US Food & Drug Administration. (2023, July 6). FDA Converts Novel Alzheimer’s Disease Treatment to Traditional Approval. https://www.fda.gov/news-events/press-announcements/fda-converts-novel-alzheimers-disease-treatment-traditional-approval
van Dyck, C. H., Swanson, C. J., Aisen, P., Bateman, R. J., Chen, C., Gee, M., Kanekiyo, M., Li, D., Reyderman, L., Cohen, S., Froelich, L., Katayama, S., Sabbagh, M., Vellas, B., Watson, D., Dhadda, S., Irizarry, M., Kramer, L. D., & Iwatsubo, T. (2023). Lecanemab in Early Alzheimer’s Disease. New England Journal of Medicine, 388(1), 9–21. https://doi.org/10.1056/NEJMoa2212948
