- This study characterized discontinuation and reinitiation of GLP-1 medications for a large population of patients with overweight or obesity who started a GLP-1 medication between 2018-2023. The study also identified factors associated with stopping and subsequently reinitiating – including weight change – the medications.
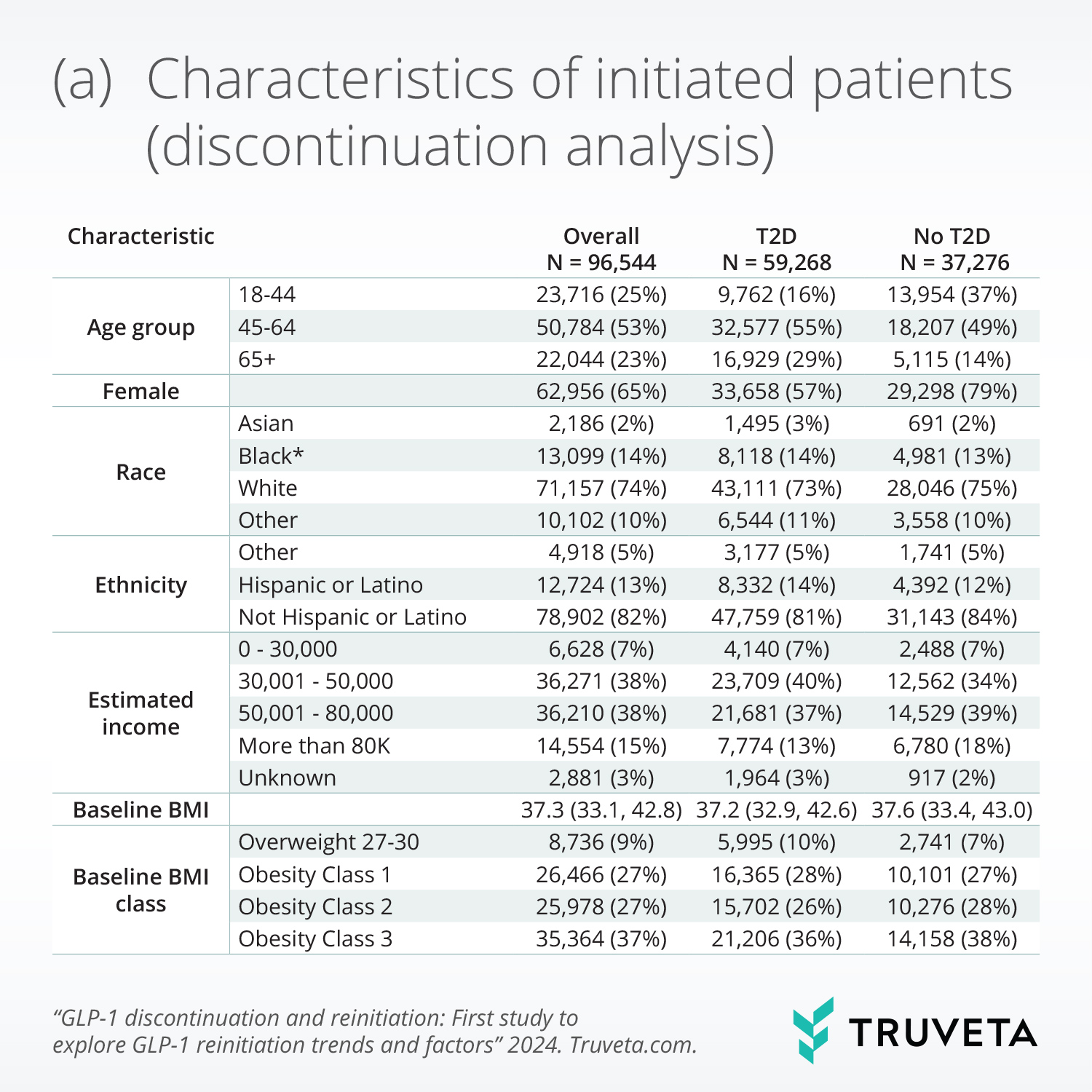
- Of the 96,544 patients initiating a GLP-1 RA medication, 46% of patients with and 65% without type 2 diabetes (T2D) discontinued within 1 year. Weight loss, income, gastrointestinal adverse events, and comorbidities were significantly associated with discontinuation.
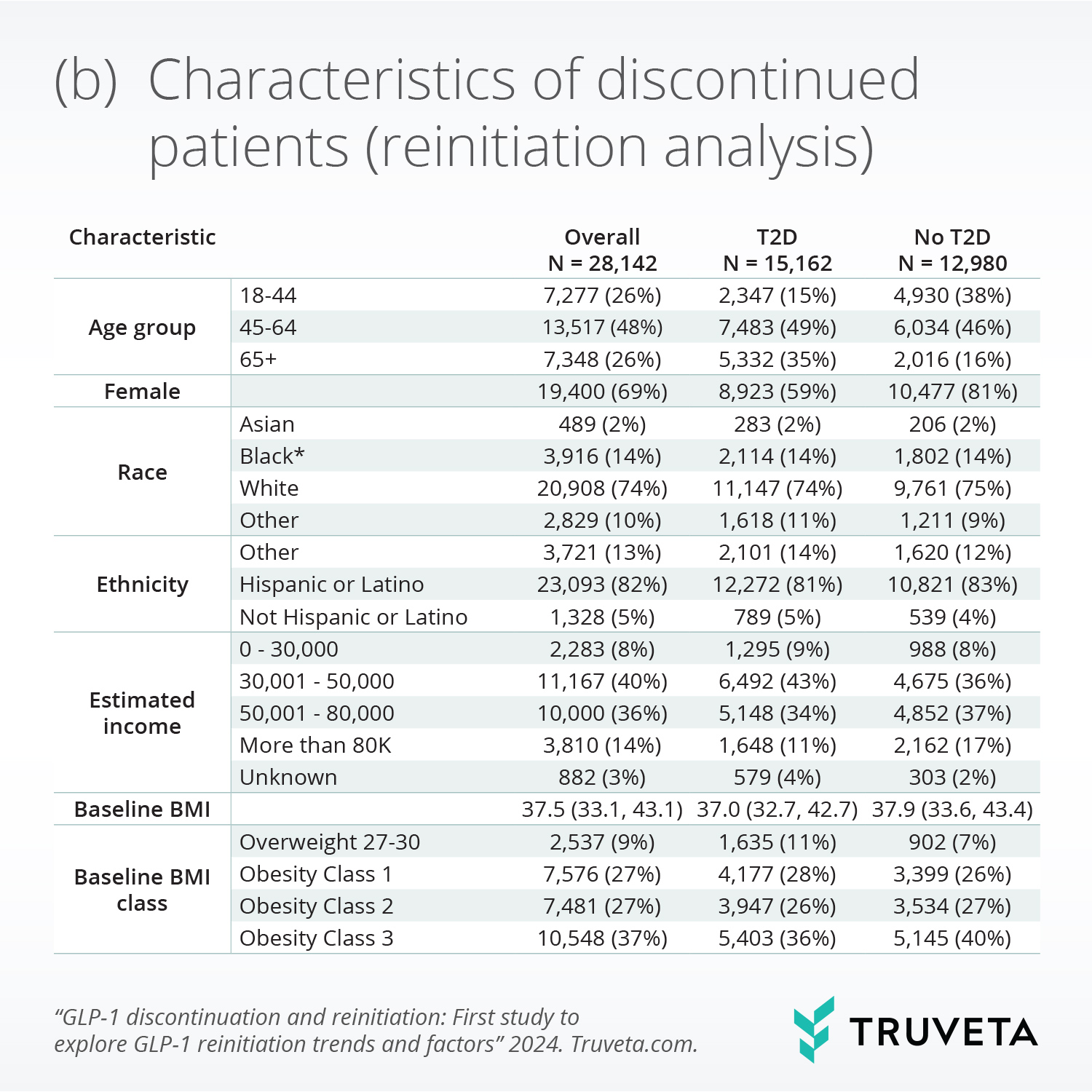
- Following discontinuation, 51% of patients with and 35% without T2D reinitiated within a year. Weight re-gain since discontinuation was significantly associated with reinitiation.
While some existing studies have explored discontinuation rates, they’ve identified widely varying rates of GLP-1 RA discontinuation at one year – and factors such as cost, insurance, comorbidities and absence of type 2 diabetes (T2D) have been associated with that discontinuation. The role of weight loss in discontinuation is not well understood. To our knowledge, no studies have characterized reinitiation of GLP-1 medications in the US.
In collaboration with Ezekiel Emanuel, MD, PhD — Vice Provost for Global Initiatives, the Co-Director of the Healthcare Transformation Institute, and the Diane v.S. Levy and Robert M. Levy University Professor at the University of Pennsylvania Perelman School of Medicine – Truveta Research shares a new study that is the first to explore GLP-1 reinitiation trends and factors among patients with overweight or obesity following discontinuation.
Key findings
Patients’ mean age at initiation was 55.1 (13.3) years, 65.2% were female, 73.7% were white, and 61.3% had T2D. Among patients without T2D, 57.2% had Individual income over $50,000 a year, compared to 49.7% of patients with T2D.
Discontinuation trends and factors
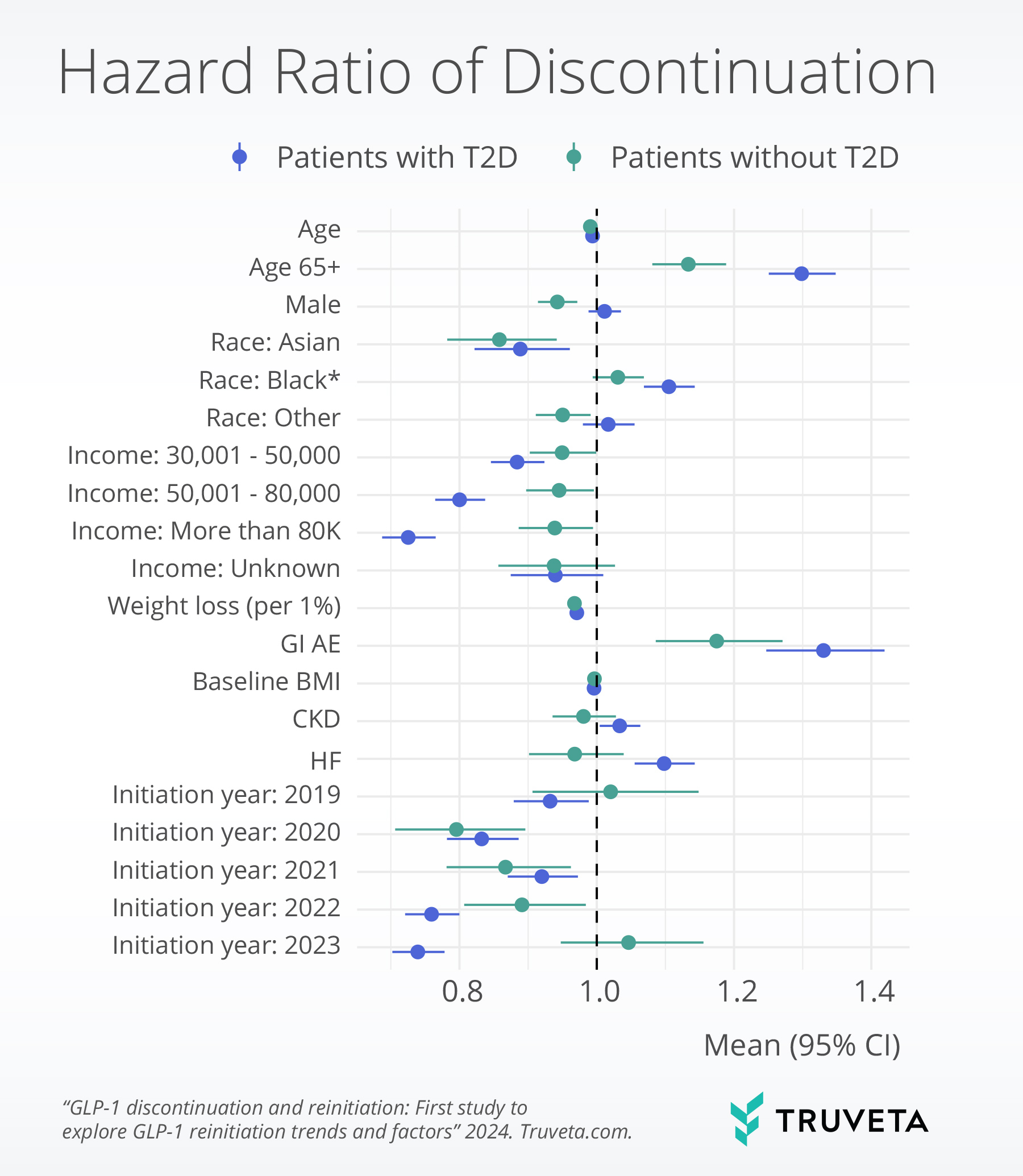
Weight loss, income, gastrointestinal adverse events, and comorbidities were significantly associated with discontinuation. Specifically, higher weight loss, absence of moderate or severe gastrointestinal adverse events, and higher income (only in the type 2 diabetes population) were significantly associated with lower discontinuation.
Reinitiation trends and factors
Weight gain since stopping GLP-1 was significantly associated with higher reinitiation, while presence of any moderate or severe gastrointestinal adverse events during initial treatment was significantly associated with lower reinitiation.
Perspective from authors
The full study — including all findings, methodologies, limitations, and more — is available on MedRxiv.

