Authors: Patricia J. Rodriguez, PhD MPH ⊕Truveta, Inc, Bellevue, WA, Brianna M. Goodwin Cartwright ⊕Truveta, Inc, Bellevue, WA, Samuel Gratzl PhD ⊕Truveta, Inc, Bellevue, WA, Charlotte Baker DrPH MPH CPH ⊕Truveta, Inc, Bellevue, WA, Nicholas Stucky, MD PhD ⊕Truveta, Inc, Bellevue, WA
- Biosimilar switching was uncommon during 2023, but increased significantly in 2024. 86.9% of all switches to biosimilars occurred in January 2024 or later.
- The CVS formulary change to remove bio-originator Humira from formularies appears to have accelerated biosimilar uptake. 77% of patients switching to biosimilar after the announcement switched to Hyrimoz, the biosimilar covered by CVS.
This blog is an extension of our poster presented at the Annual Conference for the International Society of Pharmacoepidemiology, titled Real-World Patterns in Treatment Switching to Biosimilar Adalimumab in the United States.
Adalimumab (Humira) is a biologic medication used to treat a variety of inflammatory conditions, such as arthritis (e.g., rheumatoid, psoriatic, juvenile idiopathic, and ankylosing spondylitis), psoriasis, Crohn’s disease, and ulcerative colitis. For over 20 years, Humira had market exclusivity in the US, facing no direct competition. During this period of exclusivity, the price of Humira more than doubled with monthly list prices reaching approximately $7,000 per month (Dickson et al., 2023; Rind et al., 2023; Wingrove, 2023). Humira is the highest grossing medication in history (Gibbons et al., 2023).
Market exclusively for Humira ended in January 2023, when the first biosimilar medication, adalimumab-atto (Amjevita), was introduced. Ten biosimilars have been approved in the US as of this study publication (Figure 1). Biosimilars are like generics; however, unlike generics, biosimilars are not required to be identical to the brand-name medication because biologic medications are not exactly replicable. Rather, biosimilar medications must demonstrate they are highly similar to the original medication, meaning only minor differences in clinically inactive components exist.
The introduction of competition from lower-priced biosimilars was expected to result in high levels of switching from bio-originator (brand-name) to biosimilar versions of Humira and overall price reductions. However, sales data has suggested low uptake of biosimilars, despite lower prices (Cohen, 2023). To encourage switching, CVS – one of the largest pharmacy benefit managers (PBMs) in the US – announced in January 2024 that bio-originator Humira would be removed from some formularies in April 2024, and the biosimilar Hyrimoz would be covered by all formularies (Becker, 2024).
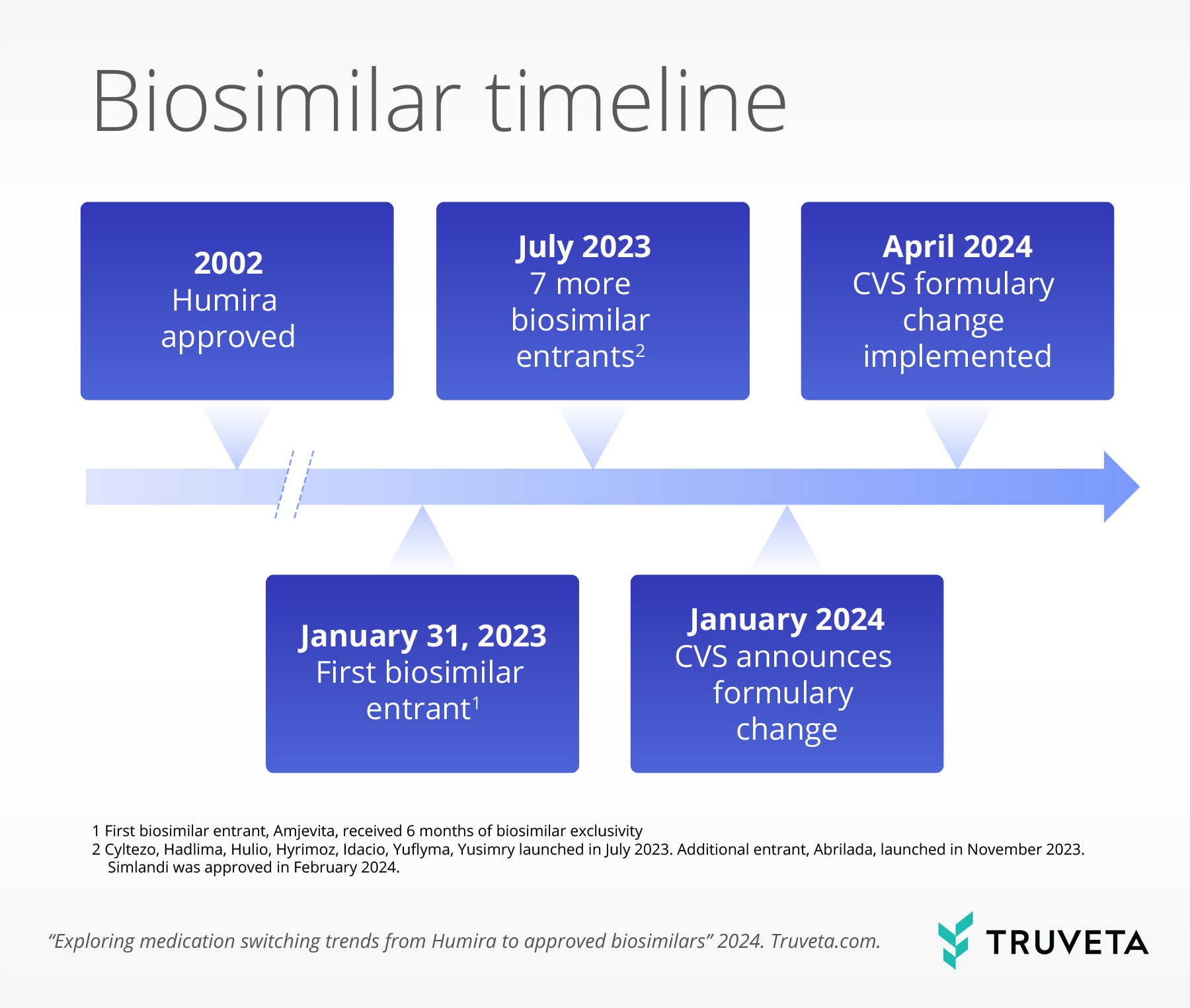
Truveta Research assessed the switching trends from bio-originator Humira to approved biosimilars before and after the CVS formulary change.
You can also view this study directly in Truveta Studio.
Methods
We describe the number and percentage of eligible patients with evidence of bio-originator to biosimilar switching, defined as a new prescription or medication dispense for any biosimilar version of Humira among the population previously using bio-originator Humira. The rate of switching per month was calculated as the number of patients with evidence of first-time switching divided by the number of patients potentially eligible to switch – those with an encounter that month who had not yet switched. Rates were scaled per 1,000 patients.
We modeled biosimilar switching over time using a Poisson regression model with an offset for the number of eligible patients. Poisson regression models are used for count data (the number of patients switching each month) and offsets account for differences in the underlying population size (eligible patients in each month). We included a primary time term (e.g., slope) for month and tested the effect of additional time terms (e.g., changes in slope) after the January 2024 announcement and after the April 2024 implementation.
Results
Compared to the cohort overall, patients who switched had similar prevalence of psoriasis (24% in both groups; p = 0.6), and higher rates of rheumatoid arthritis (35% of those who switched vs. 32% overall; p = 0.004), Crohn’s disease (21% vs. 16%; p < 0.01), psoriatic arthritis (17% vs. 14%; p = <0.001), ankylosing spondylitis (12% vs. 8%; p = <0.01), and ulcerative colitis (12% vs. 9%; p <0.01). Conditions were not mutually exclusive.
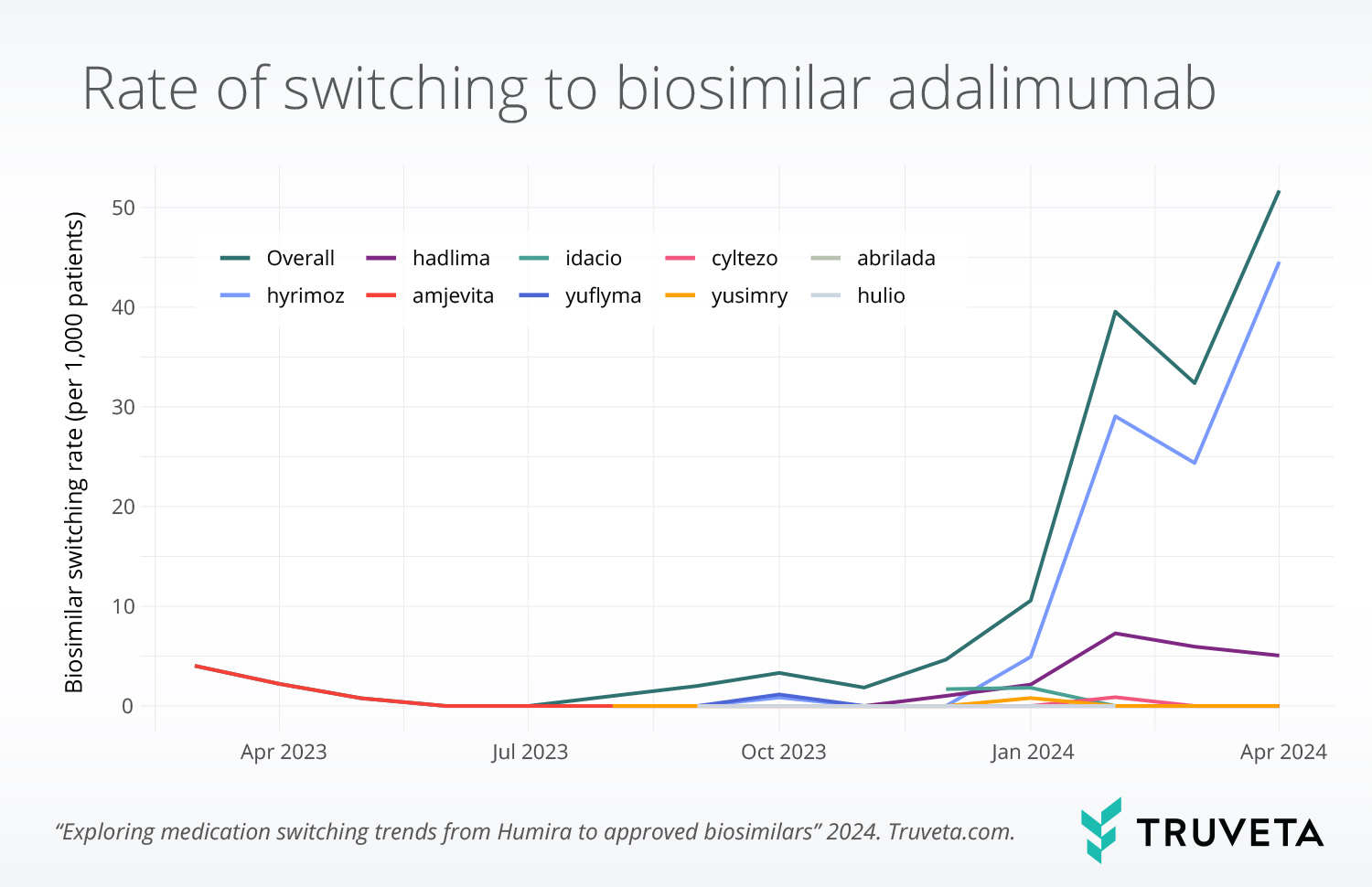
The increased switching in 2024 was driven largely by increased switching to Hyrimoz (biosimilar covered by all CVS formularies), which accounted for 76.7% of all switches during this period.
Discussion
In total, nine biosimilars were available during the study period, of which three (Abrilada, Cyltezo, Yuflyma) were designated as interchangeable with Humira, meaning the biosimilar can be substituted at the pharmacy without approval from the prescribing physician. Interestingly, switching to interchangeable versions of the drug remained low, whereas switching to the version favored by CVS formularies was high. These findings suggest that cost and insurance coverage may play an important role in switching, with interchangeability being a less important driver. However, more robust analysis is needed.
As with all research, this analysis was subject to limitations. First, the analysis did not consider which biosimilars were approved for which conditions, but rather included all patients on bio-originator Humira. Second, all patients with an outpatient office or telehealth visit in the given month were included. However, some of these visits may have been unrelated to Humira use and may not represent genuine opportunities for switching. Further, prescriptions written at health systems outside Truveta are not captured in this analysis, though dispensing at outside health systems is included.
Pricing and availability of biosimilars will likely continue to change in the coming months. Ongoing analysis is needed to study the relative influence of increased competition, pricing decisions, formulary coverage, and interchangeability designations. Future research should also consider long-term outcomes for patients switching to biosimilars. The data used in this study were extracted on May 28, 2024.
Citations
Cohen, J. (2023, December 6). Humira Biosimilars Not Gaining Traction Epitomizes Dysfunctional U.S. System. Forbes. https://www.forbes.com/sites/joshuacohen/2023/12/04/humira-biosimilars-not-gaining-traction-epitomizes-dysfunctional-us-system/
Dickson, S. R., Gabriel, N., & Hernandez, I. (2023). Contextualizing the Price of Biosimilar Adalimumab Based on Historical Rebates for the Original Formulation of Branded Adalimumab. JAMA Network Open, 6(7), e2323398. https://doi.org/10.1001/jamanetworkopen.2023.23398
Gibbons, J. B., Laber, M., & Bennett, C. L. (2023). Humira: The first $20 billion drug. The American Journal of Managed Care, 29(2), 78–80. https://doi.org/10.37765/ajmc.2023.89315
Rind, D., Agboola, F., Nikitin, D., McKenna, A., Nhan, E., Seidner, M., & Pearson, S. (2023). Unsupported Price Increase Report: Unsupported Price Increases Occurring in 2022. https://icer.org/wp-content/uploads/2023/12/UPI_2023_Report_121123.pdf
Wingrove, P. (2023, February 1). Focus: AbbVie’s Humira gets a U.S. rival, but costs could stay high. Reuters. https://www.reuters.com/business/healthcare-pharmaceuticals/abbvies-humira-gets-us-rival-costs-could-stay-high-2023-01-31/