Authors: Brianna M. Goodwin Cartwright, MS ⊕Truveta, Inc, Bellevue, WA, Patricia J. Rodriguez, PhD MPH ⊕Truveta, Inc, Bellevue, WA, Samuel Gratzl, PhD ⊕Truveta, Inc, Bellevue, WA, Charlotte Baker, DrPH MPH CPH ⊕Truveta, Inc, Bellevue, WA, Duy Do, PhD ⊕Truveta, Inc, Bellevue, WA, Nicholas Stucky, MD PhD ⊕Truveta, Inc, Bellevue, WA
- Increasing demand for GLP-1 RA medications has led to global shortages, which could result in patients switching to other medications, or discontinuing their treatment completely. In this study, Truveta Research explores patterns of GLP-1 RA switching from 2018 to 2023 and demographic characteristics of patients who switched.
- Overall, 10.9% of GLP-1 RA users in this study switched to a different GLP-1 RA medication at some point within the study period.
- Switching rates were highest in January 2023 (around 3% for the study population regardless of the presence of type 2 diabetes (T2D) or overweight/obesity).
- A large portion of the study population switched from dulaglutide labeled for T2D (26.1%) and from semaglutide labeled for T2D (21.0%). Among patients who switched to a different GLP-1 RA medication, they primarily switched to semaglutide labeled for T2D (24.5%) or to tirzepatide labeled for T2D (14.9%).
This blog is an extension of our poster presented at the Annual Conference for the International Society of Pharmacoepidemiology, titled Real-World Patterns in GLP-1 RA Switching from 2018-2023 in the US.
The Centers for Disease Control and Prevention (CDC) estimates that more than 40% of US adults have obesity and 11% have diabetes (National Center for Health Statistics, 2023; Centers for Disease Control and Prevention, 2024). Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are a class of drugs approved for treatment of type 2 diabetes (T2D), overweight, and obesity.
The increased demand for GLP-1 RAs has been primarily driven by their clinically proven effectiveness in diabetes, weight loss, and cardiovascular disease management (Yao et al., 2024; Wilding et al., 2021; Lincoff et al., 2023). In recent years, GLP-1 RA use has expanded to people with lower A1C and BMI levels at the time of prescription initiation and surged in popularity among individuals with both T2D and overweight/obesity (Gratzl et al., 2024). The increasing demand has, in part, contributed to global shortages (Putka, 2023; Whitley et al., 2023). In England, estimates of up to 400,000 people have been affected by the GLP-1 RA shortage (Seth, 2024).
Little is known about whether the global shortages have prevented patients from accessing their prescribed anti-diabetic (ADM) or anti-obesity (AOM) medications or switching to other GLP-1 RA medications. The objective of this study was to describe the temporal patterns of GLP-1 RA switching from 2018 to 2023, as well as demographic characteristics of patients who switched.
You can also view this study directly in Truveta Studio.
Methods
Using a subset of Truveta Data, we identified patients with any medication dispense (e.g., medication fill) of a GLP-1 RA (semaglutide AOM, semaglutide ADM oral, semaglutide ADM injectable, liraglutide ADM, exenatide ADM, lixisenatide ADM, tirzepatide ADM, and dulaglutide ADM) between January 2018 and October 2023. Patients were excluded if they did not have at least two months of GLP-1 RA prescription fills. Brand names were used to infer labeled indication (AOM or ADM) and route of administration (injectable or oral). A medication was excluded from the analysis if the brand name was unavailable. Medication dispense data includes fills for prescriptions written both within and outside Truveta’s constituent health care systems, providing greater observability into patients’ medication history. Medication dispense histories are updated at each encounter, and include fill dates, NDC or RxNorm codes, quantity dispensed, and days of medication supplied.
For dispense data with a supply greater than 30 days, patients were assumed to have supply on hand for subsequent months (i.e., for supply of 60- or 90-days patients were assumed to have supply for 2 or 3 months, respectively). Switching was defined as when a patient filled a different GLP-1 RA drug during two sequential months. We describe the drugs and months with the highest rates of switching.
Results are presented overall for all GLP-1 users and stratified by populations with overweight/obesity (BMI >= 27) or T2D. We identified patients as having a previous T2D diagnosis or having overweight/obesity (BMI >=BMI) using either diagnosis codes or BMI measurements.
Results
Population
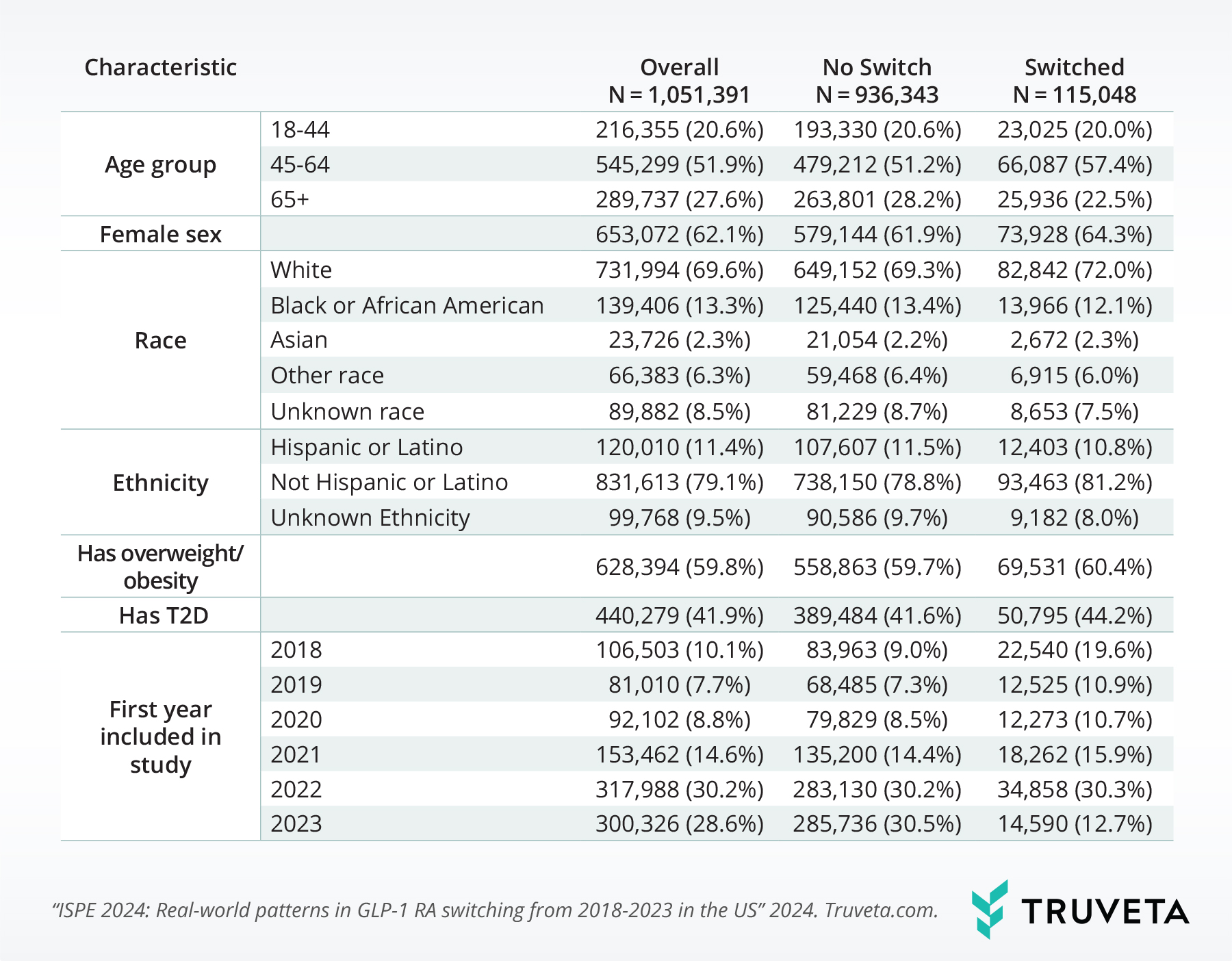
This study included 1,051,391 people. Most individuals were 45-64 years old (51.9%), of female sex (62.1%), white race (69.6%), and not Hispanic or Latino (79.1%).
About 59.8% of the study sample had overweight/obesity and 41.9% had T2D.
Overall, 10.9% of the study population switched GLP-1 RA at some point within the study period. The population who switched were made up of a higher percentage of people in the 45-64 age range, female, white, not Hispanic or Latino, those with overweight/obesity, T2D, and those who initiated treatment before 2021.
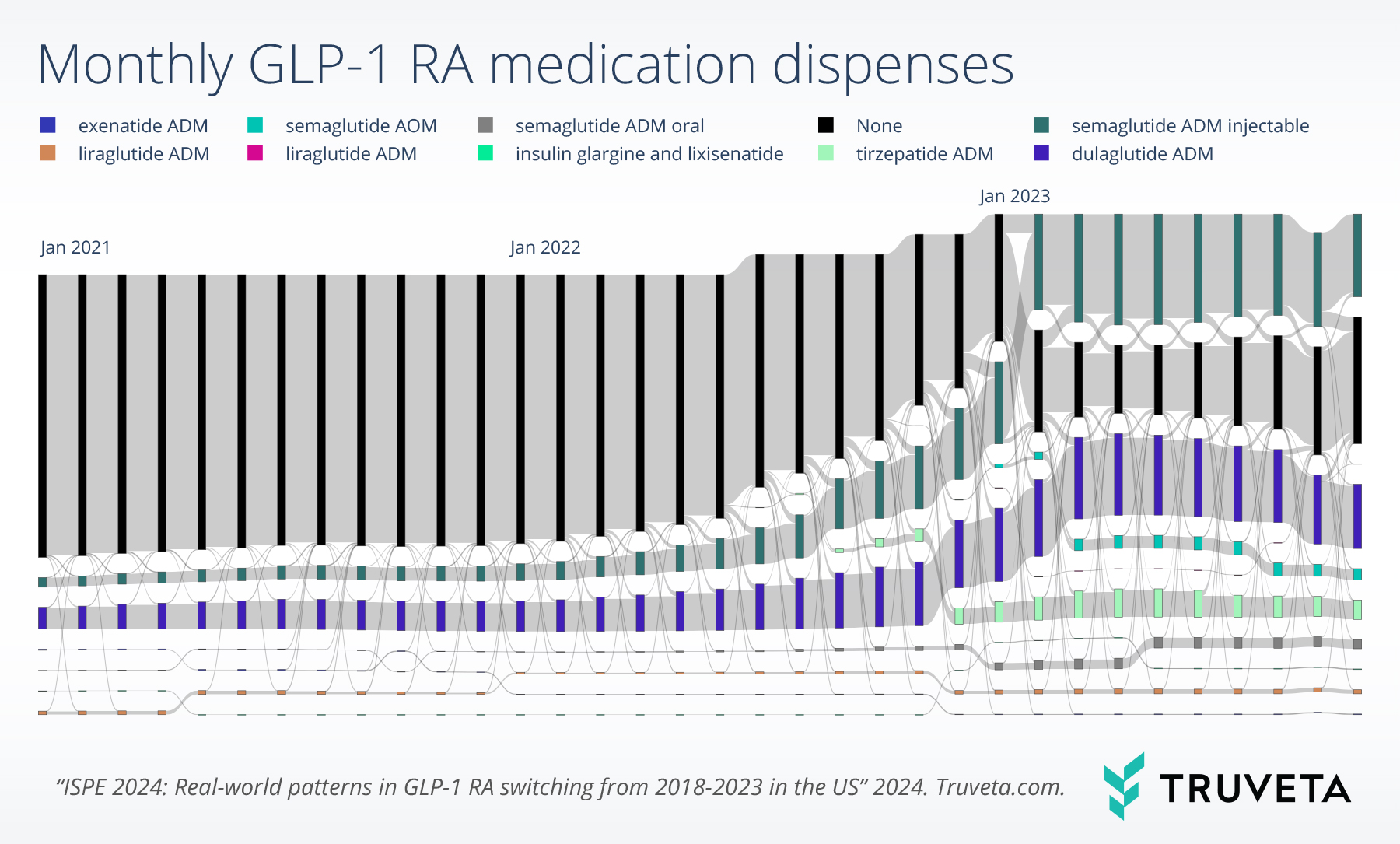
Switching
Switching rates were highest in January 2023 (3.0%, 3.0%, and 3.1% of the overall, T2D, and overweight/obesity populations, respectively).
Among patients who switched to a different GLP-1 RA medication, many of them switched from dulaglutide labeled for T2D (26.1%) or semaglutide labeled for T2D (21.0%).
Furthermore, many patients switched to semaglutide labeled for T2D (24.5%) or tirzepatide labeled for T2D (14.9%). Similar trends were observed for both population subgroups with T2D or overweight/obesity.
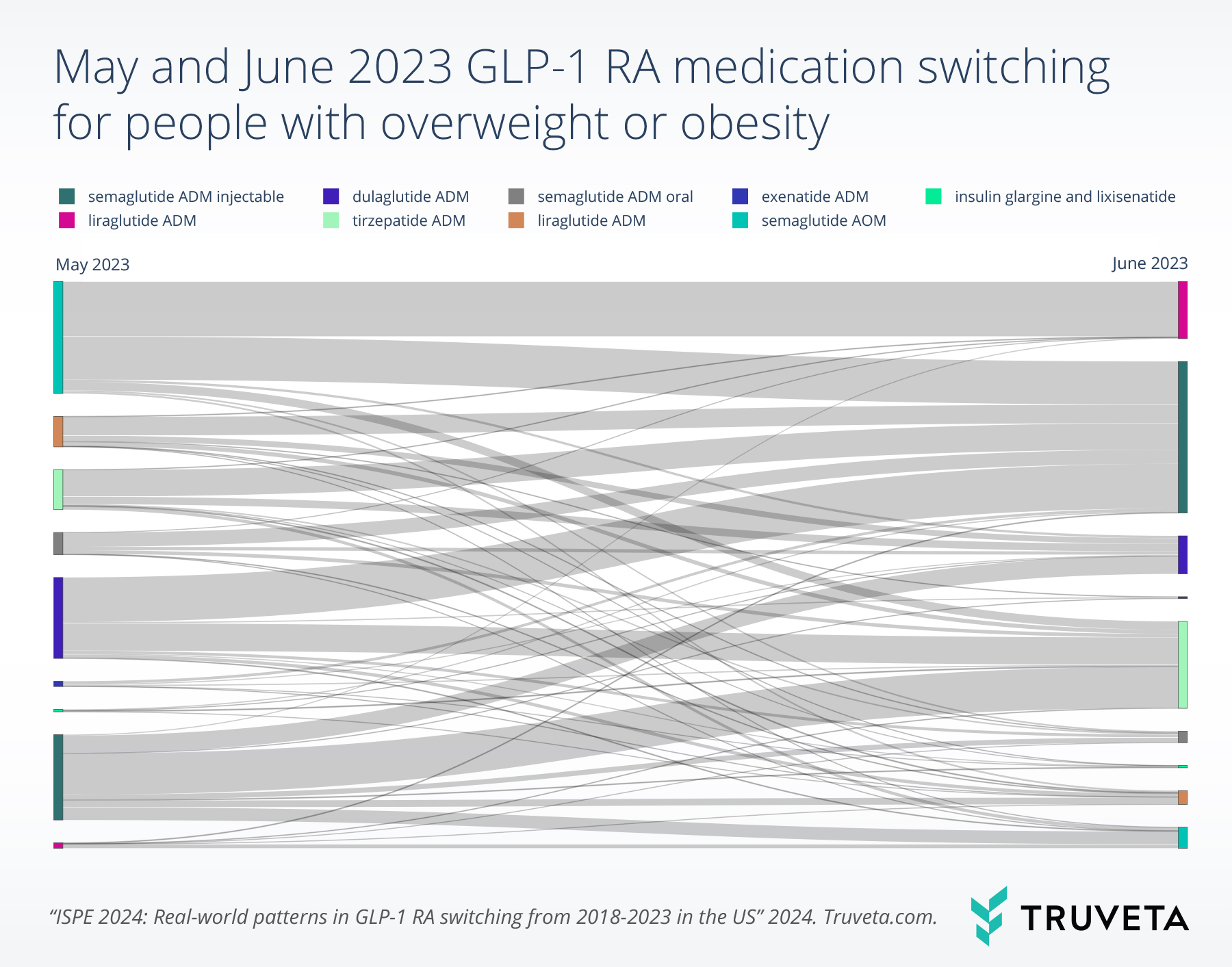
Patients with overweight/obesity experienced high rates of switching between May and June 2023 (2.6%).
Patients primarily switched from dulaglutide labeled for T2D (17.5%). Those who switched between these two months primarily switched to semaglutide labeled for T2D (28.0%) (see figure below).
Note that while some patients switched from a specific drug, other patients switched to the same drug, such as semaglutide ADM injectable.
Discussion
In light of the recent increase in GLP-1 RA use, we found that switching patterns varied by medication, with higher rates observed for medications approved in earlier years. Within certain months there were high rates of switching from and switching to semaglutide labeled for T2D, potentially pertaining to geographic and timing variations in both usage and shortages. This is consistent with anecdotal reports showing the increase in semaglutide ADM use being larger in some metropolitan cities than others, and does not always correlate with high diabetes prevalence (Trilliant Health, 2023).
There are a few limitations with this study. First, not all people included in the study had evidence in their medical records for T2D or overweight/obesity, although this may indicate off-label use and provide some visibility into this group. Second, this study did not consider discontinuation, although in another study, Truveta explored factors associated with GLP-1 RA discontinuation and reinitiation – including but not limited to weight loss/gain, adverse side-effects, comorbidities, and sociodemographic characteristics. Additional research is needed to understand whether and how shortages might have resulted in an increase in GLP-1 RA switching.
This study highlights many areas for future research. For example, strategies employed by patients to cope with global shortages in GLP-1 RAs – either by switching to a different medication or reducing the dosage to make their medication last longer – could potentially dampen the effectiveness of the medication. Furthermore, understanding GLP-1 RA switching rates in conjunction with regional variation in shortages and social drivers of health (SDOH) would be beneficial.
Data are constantly changing and updating. These findings are consistent with data accessed on January 29, 2024.
Citations
Centers for Disease Control and Prevention. (2024, March 15). National Diabetes Statistics Report. https://www.cdc.gov/diabetes/php/data-research/index.html
Yao H, Zhang A, Li D, Wu Y, Wang CZ, Wan JY, Yuan CS. (2024). Comparative effectiveness of GLP-1 receptor agonists on glycemic control, body weight, and lipid profile for type 2 diabetes: systematic review and network meta-analysis. BMJ, 384. https://doi.org/10.1136/bmj-2023-076410
Wilding JP, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, McGowan BM, Rosenstock J, Tran MT, Wadden TA, Wharton S. (2021). Once-weekly semaglutide in adults with overweight or obesity. New England Journal of Medicine, 384(11):989-1002. https://doi.org/10.1056/NEJMoa2032183
Lincoff AM, Brown-Frandsen K, Colhoun HM, Deanfield J, Emerson SS, Esbjerg S, Hardt-Lindberg S, Hovingh GK, Kahn SE, Kushner RF, Lingvay I. (2023). Semaglutide and cardiovascular outcomes in obesity without diabetes. New England Journal of Medicine, 389(24):2221-32. https://doi.org/10.1056/NEJMoa2307563
Centers for Disease Control and Prevention. (2024, March 15). National Diabetes Statistics Report. https://www.cdc.gov/diabetes/php/data-research/index.html
Gratzl, S., Rodriguez, P. J., Cartwright, B. M. G., Baker, C., Do, D., & Stucky, N. L. (2024). Monitoring Report: GLP-1 RA Prescribing Trends – March 2024 Data. https://doi.org/10.1101/2024.01.18.24301500
National Center for Health Statistics. (2023, January 5). Obesity and Overweight. https://www.cdc.gov/nchs/fastats/obesity-overweight.htm
Putka, S. (2023, January 24). Diabetes Patients Also Struggle to Access GLP-1 Agonists. MedpageToday. https://www.medpagetoday.com/special-reports/features/102773
Seth, S. (2024, January 25). Diabetes patients struggling without “wonder drug.” https://www.bbc.com/news/uk-england-hampshire-68083710
Trilliant Health. (2023). 2023 Trends Shaping the Health Economy Value for Money. https://www.trillianthealth.com/hubfs/TH_Annual%20Report_2023.10.25%20(2).pdf?hsCtaTracking=e0a5d398-f873-4e1f-b574-54c459b40597%7C64ca9886-cf47-4a6f-9a43-814418eb9864
Whitley, H. P., Trujillo, J. M., & Neumiller, J. J. (2023). Special Report: Potential Strategies for Addressing GLP-1 and Dual GLP-1/GIP Receptor Agonist Shortages. Clinical Diabetes, 41(3), 467–473. https://doi.org/10.2337/cd23-0023

