- In partnership with Reuters, Truveta Research explored the percentage of patients who received a first-time diagnosis of type 2 diabetes, cardiovascular disease, or sleep apnea within 15 days of their first-time GLP-1 prescriptions.
- Truveta Research found that for every 1,000 patients with a first-time GLP-1 prescription, 42 were diagnosed with type 2 diabetes within 15 days in 2024, up from 32 in 2020. Over the same period, the number of sleep apnea diagnoses per 1,000 patients rose to 11 from 8 and the number of cardiovascular disease diagnoses increased to 15 from 13.
- The study also found that the likelihood of receiving a first-time diagnosis of T2D or sleep apnea within 15 days of initiating GLP-1 RAs increased with higher overweight/obesity classes. For instance, individuals with Class 3 obesity were 2.1 times more likely to receive a first-time T2D diagnosis and 3.2 times more likely to receive a sleep apnea diagnosis compared to those with overweight.
The rise of glucagon-like peptide-1 receptor agonists (GLP-1 RA) drugs has transformed the healthcare landscape, capturing widespread attention and adoption. According to a recent KFF Health Tracking Poll, one in eight adults in the U.S. has taken a GLP-1 RA medication (Montero et al., 2024), highlighting their remarkable popularity. Our team has also shown increased GLP-1 RA prescribing (Gratzl et al., 2024). Originally developed for managing type 2 diabetes, these medications have expanded their indications to include obesity and cardiovascular disease, broadening their appeal and utility (U.S. Food & Drug Administration, 2019, 2023, 2024). Further, recent results have shown one GLP-1 RA may reduce the severity of obstructive sleep apnea (Malhotra et al., 2024). This rapid expansion underscores the growing role GLP-1 RAs play in addressing complex health issues.
In previous analyses, we uncovered an intriguing trend: individuals with type 2 diabetes who are initiating treatment of GLP-1 RA therapies are increasingly doing so at lower A1cs and BMIs (Cartwright et al., 2024). This shift in usage patterns suggests these drugs are no longer solely reserved for managing severe metabolic conditions but are becoming a broader tool in preventive strategies. Their versatility raises important questions about how these treatments are reshaping patient behaviors and treatment practices.
We wondered how these drugs may play a role in treatment patterns, particularly in populations with overweight or obesity. Could evolved care-seeking behaviors be prompting an increase in first-time diagnoses or changing the way patients engage with the healthcare system? We worked exclusively with Reuters to better understand how first-time diagnosis patterns for people within 15 days of their first GLP-1 RA prescription and understand how this may have changed over time. You can also view this full study in Truveta Studio.
Methods
We included a population of people with a first-time GLP-1 RA prescription between January 2018 and October 2024. Patients were required to have their most recent BMI over 27 (indicating having overweight or obesity, and the lowest BMI indicated for treatment with GLP-1 RA). Patients were also required to have at least one outpatient encounter within the four years prior the prescription.
Within the study period, we described the percentage of people who received a first-time type 2 diabetes (T2D), cardiovascular disease (CVD), or sleep apnea diagnosis within 15 days of their GLP-1 prescription. We looked at these rates across overweight/obesity classes.
We also investigated changes over time in the rates between January 2018 and October 2024.
Results
We included a population of 711,783 people with a first-time GLP-1 RA prescription who have overweight or obesity. The population primarily had obesity (Class 1 [BMI 30-35]: 30.9%, Class 2 [BMI 35-40]: 26.2%, and Class 3 [BMI 40+]: 31.5%) compared to overweight (BMI 27-30: 11.3%).
Rate of first-time prescriptions
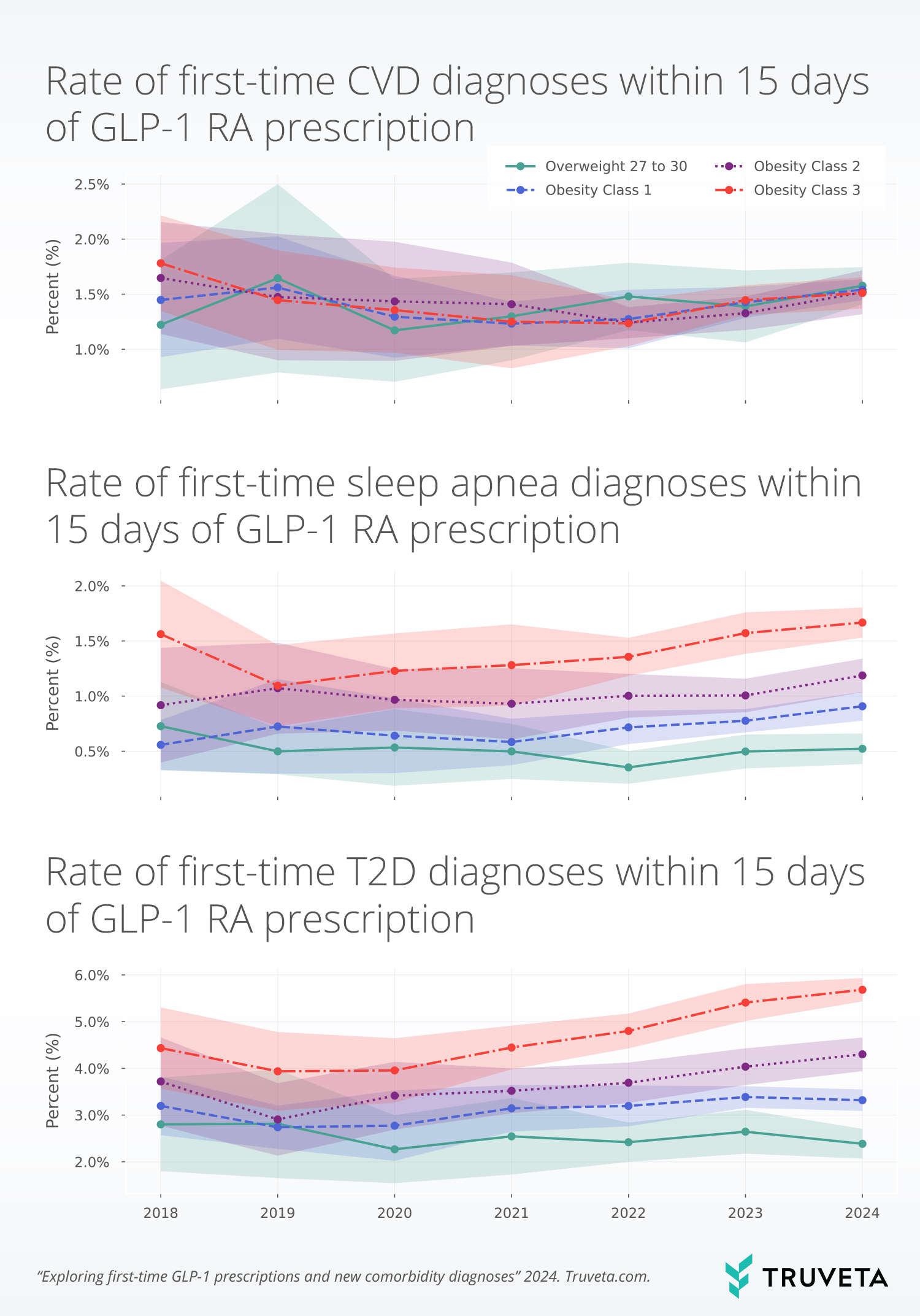
The table below shows the percentage of the population who received a first-time diagnosis of T2D, CVD, or sleep apnea within 15 days of their first-time GLP-1 prescriptions. The rate of first-time diagnoses increased with increasing overweight/obesity classes for T2D and sleep apnea.
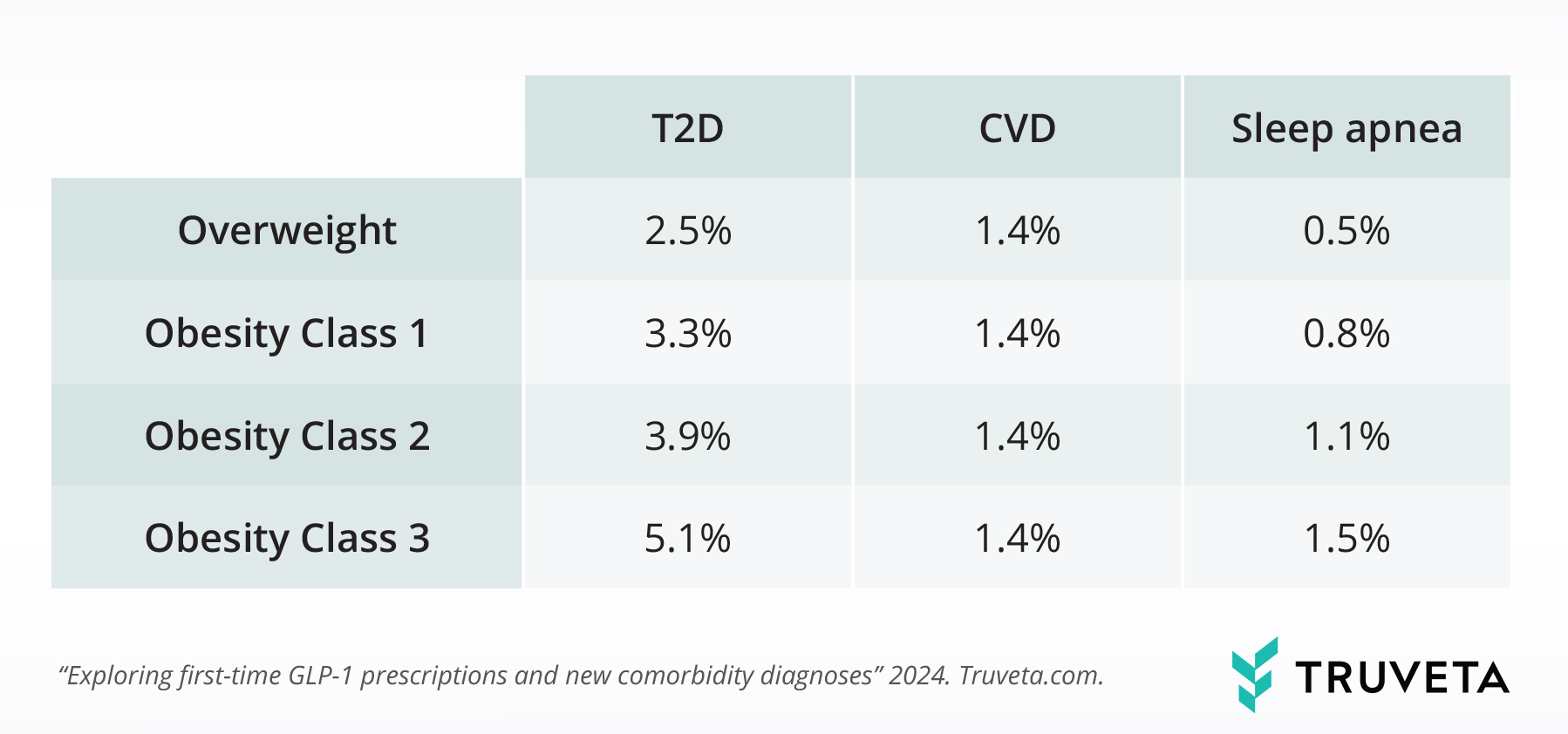
Across the study period, the rate of first-time sleep apnea diagnoses within 15 days of the prescription was 3.2x higher for people with Class 3 obesity compared to those with overweight (BMI 27-30). The rate of first-time T2D diagnoses within 15 days of a GLP-1 prescription was 2.1x higher for people with Class 3 obesity compared to those with overweight (BMI 27 – 30).
Trends in first-time diagnoses over time
The rate of first-time CVD diagnoses increased by 14.3%; however, it should be noted that this was not a large increase, as it was only an increase of 0.2 percentage points between 2020 to 2024 (1.3% to 1.5%). That means that for every 1,000 patients with a first-time GLP-1 prescription, 15 were diagnosed with CVD within 15 days in 2024, up from 13 in 2020. The largest increases in first-time CVD diagnosis following first-time GLP-1 RA prescription were seen in the group of people having overweight or BMIs between 27 to 30 (34.5% increase from 2020 to 2024).
Overall, sleep apnea increased by 34%, from 0.8% in 2020 to 1.1% in 2024. That means that for every 1,000 patients with a first-time GLP-1 prescription, 11 were diagnosed with sleep apnea within 15 days in 2024, up from 8 in 2020. For most years, increasing overweight/obesity classes correlated with increased first-time diagnosis rates. Although all obesity classes increased in the rate of first-time sleep apnea diagnoses between 2020 and 2024, the rates did not increase linearly with obesity classes. People in Obesity Class 1 experienced the biggest increases from 2020- 2024 (41.8%), while people in Obesity Class 2 experienced the smallest increases (22.8%). The overweight class did not see an increase between 2020-2024.
The rate of first-time T2D increased by 31.6%, from 3.2% in 2020 to 4.2% in 2024. That means that for every 1,000 patients with a first-time GLP-1 prescription, 42 were diagnosed with T2D within 15 days in 2024, up from 32 in 2020. Like sleep apnea, increased rates were associated with increased overweight/obesity classes. First-time T2D diagnoses within 15 days of the first-time GLP-1 prescription increased with increasing obesity classes between 2020 and 2024 (Class 1: +19.7%, Class 2: +25.9%, Class 3: + 43.7%).
Discussion
This study provides new insights into the evolving role of GLP-1 RAs in healthcare, particularly how their use amongst people with overweight or obesity coincides with first-time diagnoses of type 2 diabetes, sleep apnea, and cardiovascular disease. The results here highlight the role of GLP-1 RAs may play as a catalyst for earlier diagnosis and intervention in metabolic and related conditions.
Our findings highlight potentially new patterns and shifts in first-time diagnoses of additional diseases following first-time GLP-1 RAs prescriptions. Notably, our previous research highlighted how individuals with T2D initiate GLP-1 RA treatment at lower A1c and BMI levels in more recent years (Cartwright et al., 2024), reflecting a trend toward broader and more accessible use of these drugs. This shift prompts critical discussions about how these therapies may be altering care-seeking behaviors and diagnosis timelines. This study revealed that the likelihood of receiving a first-time diagnosis of T2D or sleep apnea within 15 days of initiating GLP-1 RAs increased with higher overweight/obesity classes. For instance, individuals with Class 3 obesity were 2.1 times more likely to receive a first-time T2D diagnosis and 3.2 times more likely to receive a sleep apnea diagnosis compared to those with overweight. The association between GLP-1 RA initiation and first-time diagnoses may suggest that healthcare providers are actively screening for conditions like T2D and sleep apnea following the prescription. This proactive approach could facilitate earlier detection and treatment, improving patient outcomes (Kristensen et al., 2019; Sattar et al., 2021). It may also reflect patient’s increased engagement with healthcare services following GLP-1 RA prescriptions. Providers could leverage this window to optimize patient evaluations, address unmet diagnostic needs, and establish comprehensive care plans. In this study, we did not compare to a population who was not receiving GLP-1 RA prescriptions; therefore, these trends may be increasing for all people (not just people with first-time GLP-1 RA prescriptions).
From 2020 to 2024, rates of first-time T2D and sleep apnea diagnoses increased, particularly among individuals with obesity. The largest increases in T2D diagnosis were observed in Class 3 obesity, while sleep apnea diagnosis rates saw the largest proportional growth in Class 1 obesity.
A few limitations exist within this study. First, we required patients to have at least one outpatient encounter in the four years prior to their GLP-1 RA prescription. This was a wide lookback period as we wanted to allow patients who might now have sought care recently to be included in the population. However, this criterion allowed for the inclusion of people who may not receive usual care inside a Truveta member health system. Further, we did not confirm if the patient received care outside a Truveta health system within the study period. Additionally, people with higher BMIs have higher rates of T2D and sleep apnea; the correlation between increased first-time T2D and sleep apnea diagnoses may be due to a higher percentage of the underlying population having T2D and sleep apnea (Gray et al., 2015; Hu et al., 2014; Young, 2002). We also did not distinguish between people seeking care with GLP-1 RAs and those who were not seeking GLP-1 RA prescriptions. Care-seeking behavior may affect timing of subsequent first-time diagnoses. We also used a general sleep apnea definition, rather than using a more specific definition of obstructive sleep apnea. Finally, we did not test for statistical differences, but rather described the changes over time.
Further research is warranted to examine if additional screenings or tests occur after a patient request or is prescribed a GLP-1 RA. Further, studying the interplay between healthcare access, socioeconomic factors, and diagnosis patterns could illuminate barriers to equitable use and help refine clinical guidelines. Exploring the role of patient preferences and provider practices in driving these trends can inform strategies to maximize the benefits of GLP-1 RAs while mitigating potential overuse or misuse.
These are preliminary research findings and not peer reviewed. Data are constantly changing and updating. These findings are consistent with data accessed on November 4, 2024.
You can also view this study directly in Truveta Studio.
Citations
Cartwright, B., Rodriguez, P., Gratzl, S., Baker, C., Worsham, C., Jena, A., & Stucky, N. (2024). HSD72 Baseline A1C and BMI Trends for People with Type 2 Diabetes Receiving First-Time GLP-1 Ra Prescriptions. Value in Health, 27(6), S233. https://doi.org/10.1016/j.jval.2024.03.1295
Gratzl, S., Rodriguez, P. J., Cartwright, B. M. G., Baker, C., & Stucky, N. L. (2024). Monitoring Report: GLP-1 RA Prescribing Trends – September 2024 Data. https://doi.org/10.1101/2024.01.18.24301500
Gray, N., Picone, G., Sloan, F., & Yashkin, A. (2015). Relation between BMI and diabetes mellitus and its complications among US older adults. Southern Medical Journal, 108(1), 29–36. https://doi.org/10.14423/SMJ.0000000000000214
Hu, Y., Bhupathiraju, S. N., de Koning, L., & Hu, F. B. (2014). Duration of obesity and overweight and risk of type 2 diabetes among US women. Obesity (Silver Spring, Md.), 22(10), 2267–2273. https://doi.org/10.1002/oby.20851
Kristensen, S. L., Rørth, R., Jhund, P. S., Docherty, K. F., Sattar, N., Preiss, D., Køber, L., Petrie, M. C., & McMurray, J. J. V. (2019). Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. The Lancet Diabetes & Endocrinology, 7(10), 776–785. https://doi.org/10.1016/S2213-8587(19)30249-9
Malhotra, A., Grunstein, R. R., Fietze, I., Weaver, T. E., Redline, S., Azarbarzin, A., Sands, S. A., Schwab, R. J., Dunn, J. P., Chakladar, S., Bunck, M. C., & Bednarik, J. (2024). Tirzepatide for the Treatment of Obstructive Sleep Apnea and Obesity. New England Journal of Medicine, 391(13), 1193–1205. https://doi.org/10.1056/NEJMoa2404881
Montero, A., Sparks, G., Presiado, M., & Hamel, L. (2024). KFF Health Tracking Poll May 2024: The Public’s Use and Views of GLP-1 Drugs. Kaiser Family Foundation. https://www.kff.org/health-costs/poll-finding/kff-health-tracking-poll-may-2024-the-publics-use-and-views-of-glp-1-drugs/
Sattar, N., Lee, M. M. Y., Kristensen, S. L., Branch, K. R. H., Del Prato, S., Khurmi, N. S., Lam, C. S. P., Lopes, R. D., McMurray, J. J. V., Pratley, R. E., Rosenstock, J., & Gerstein, H. C. (2021). Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of randomised trials. The Lancet Diabetes & Endocrinology, 9(10), 653–662. https://doi.org/10.1016/S2213-8587(21)00203-5
U.S. Food & Drug Administration. (2019). FDA approves first oral GLP-1 treatment for type 2 diabetes. https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-glp-1-treatment-type-2-diabetes
U.S. Food & Drug Administration. (2023). FDA Approves New Medication for Chronic Weight Management. https://www.fda.gov/news-events/press-announcements/fda-approves-new-medication-chronic-weight-management
U.S. Food & Drug Administration. (2024). FDA Approves First Treatment to Reduce Risk of Serious Heart Problems Specifically in Adults with Obesity or Overweight. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-reduce-risk-serious-heart-problems-specifically-adults-obesity-or
Young, T. (2002). Predictors of Sleep-Disordered Breathing in Community-Dwelling AdultsThe Sleep Heart Health Study. Archives of Internal Medicine, 162(8), 893. https://doi.org/10.1001/archinte.162.8.893