
by Truveta staff | Jan 31, 2025 | Research
Discontinuation within one year was significantly higher in patients without T2D (64.8%) compared to those with T2D (46.5%). When patients experienced greater weight loss, they were less likely to discontinue the GLP-1 RA medications. Among patients with T2D, higher...

by Truveta Research | Dec 16, 2024 | Research, Research Insights
In partnership with Reuters, Truveta Research explored the percentage of patients who received a first-time diagnosis of type 2 diabetes, cardiovascular disease, or sleep apnea within 15 days of their first-time GLP-1 prescriptions. Truveta Research found that for...

by Truveta staff | Dec 6, 2024 | Data
New results released this week from the SURMOUNT-5 clinical trial comparing tirzepatide (Zepbound) to semaglutide (Wegovy) are closely aligned to Truveta Research’s GLP-1 comparative effectiveness study, initially shared more than a year ago on November 27, 2023 and...
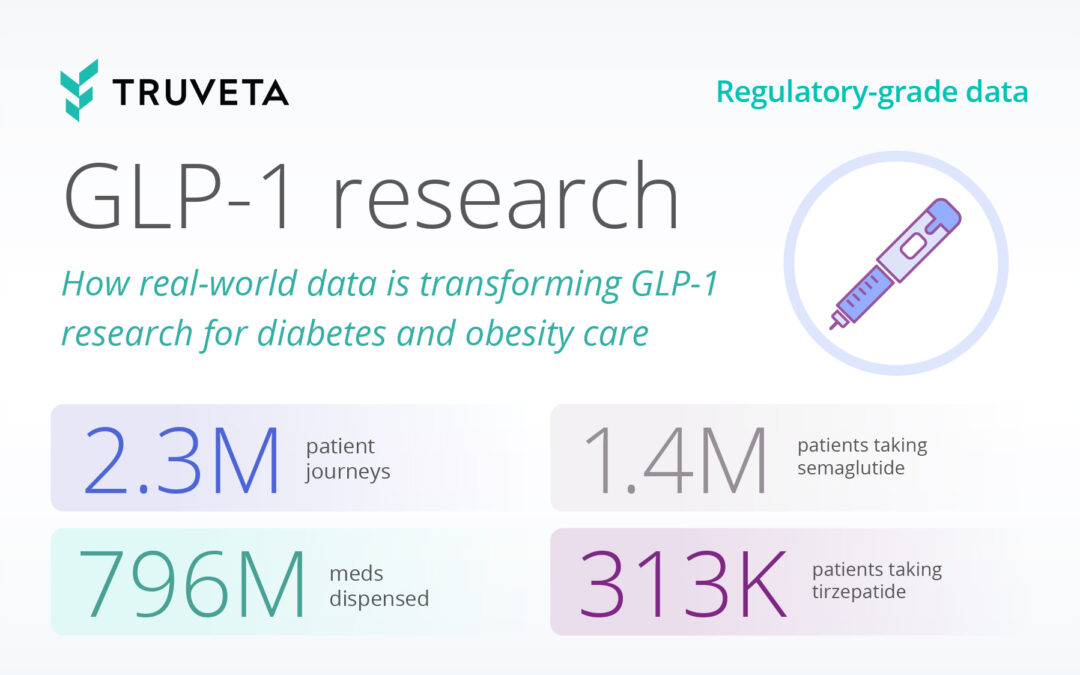
by Truveta staff | Nov 7, 2024 | Data
GLP-1 medications are revolutionizing the way we treat type 2 diabetes and obesity. First discovered forty years ago, GLP-1 receptor agonists were initially approved for type 2 diabetes in 2005, with the first weight-loss-specific approval following in 2014. Flash...
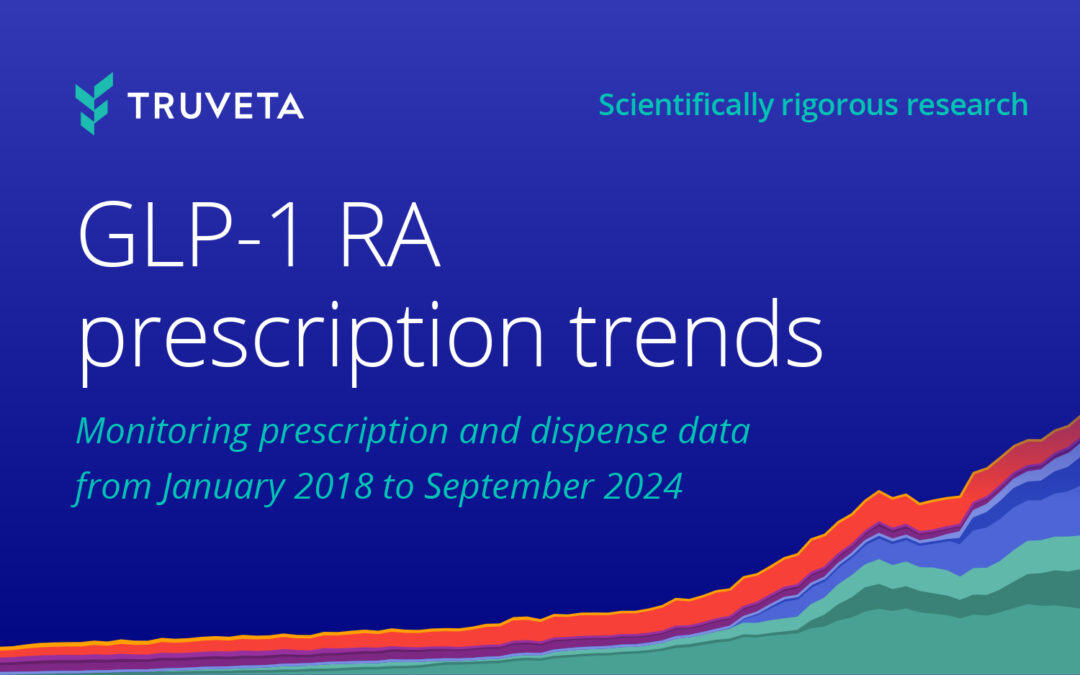
by Truveta staff | Oct 14, 2024 | Research
Overall prescribing rates for GLP-1 medications increased in September 2024 relative to June 2024 (+11.9%). While the rate of GLP-1 prescriptions labeled for type 2 diabetes were about the same as June 2024 (+4.0%), the rate of prescriptions for GLP-1 medications...






