By the time most women with ovarian cancer are diagnosed, 70-80% are already at an advanced stage of the disease. Despite declining mortality rates over the past decade, the 5-year survival rate for patients with ovarian cancer remains less than 30%. Sometimes referred to as the “silent killer” due to its insidious nature, ovarian cancer is often unnoticed or dismissed because its early symptoms can be confused with everything from irritable bowel syndrome (IBS) to menopause.
With no effective screening tools, ovarian cancer is difficult to diagnose and in urgent need of breakthrough research for the nearly 13,000 women projected to lose their lives to the condition in the United States this year. Truveta’s regulatory-grade EHR data with more than 100 million patient journeys across care settings and specialties, and the largest collection of clinical notes and images, can advance diagnostics and treatment for patients with ovarian cancer. Coming from a growing collective of 30 health systems and representing the full diversity of the United States, Truveta offers the most complete, longitudinal cancer data across the continuum of care.
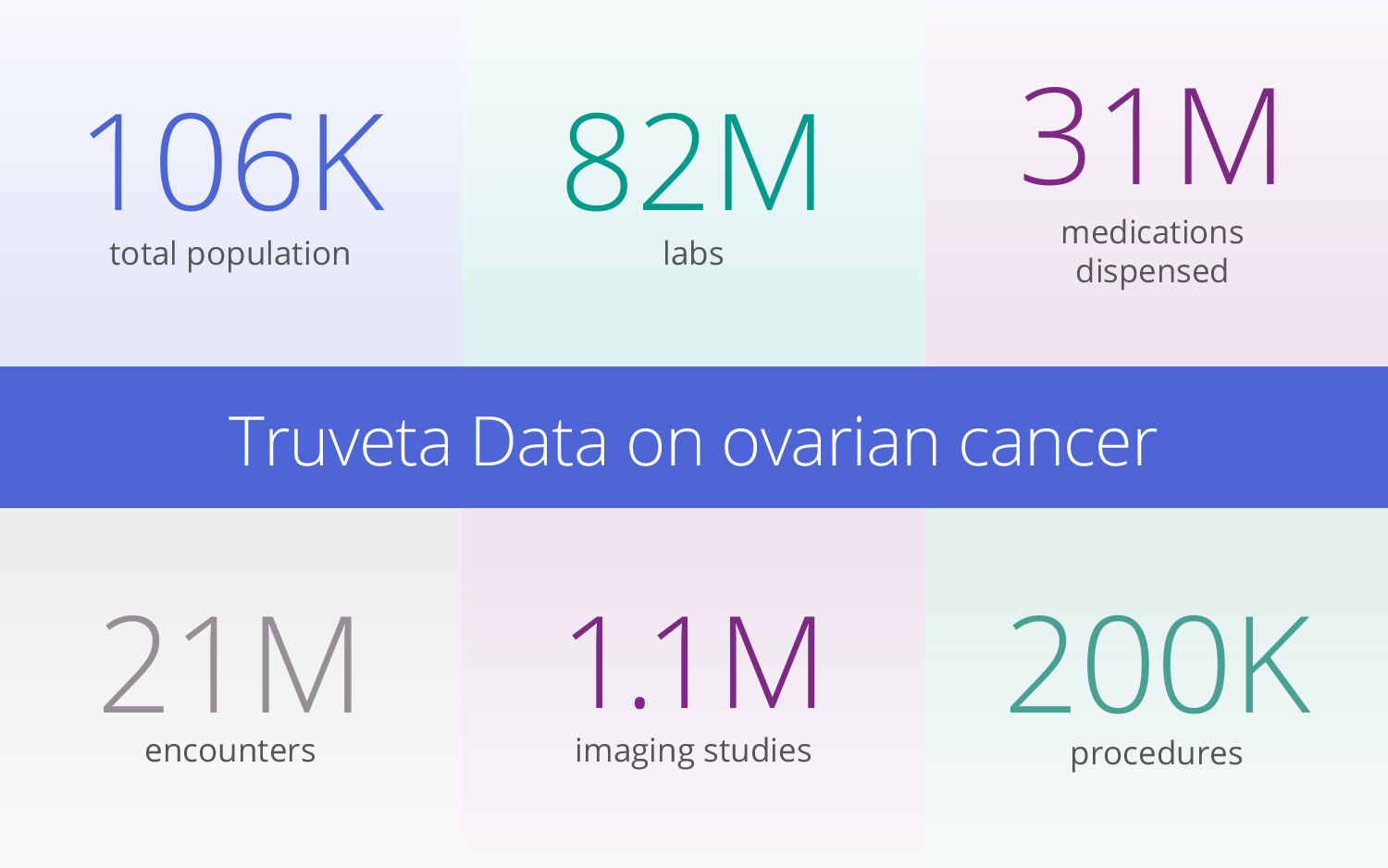
With a comprehensive view of the patient journey, researchers can gain holistic insights into ovarian cancer care to accelerate therapy development and improve patient access and outcomes. Read on to discover the depth of Truveta Data available on ovarian cancer, which includes data on roughly half of the women living with ovarian cancer in the United States.
Access complete EHR data across care settings and specialties for patients with ovarian cancer
Linked with SDOH, mortality, and claims data, Truveta’s regulatory-grade EHR data is inclusive of cancer screenings, early diagnosis and treatment outside of oncology, and oncology interactions across all stages of cancer.
With an average of 8+ years longitudinal history, researchers can gain a comprehensive view of comorbidities, all medications, symptoms prior to seeing an oncologist, specific lab results, SDOH factors, and more.
Examples of specific measures for the ovarian cancer cohort include:
- PARP inhibitors, which can lower cancer recurrence by up to 70%: 142k medication dispenses + administrations
- Mirvetuximab soravtansine-gynx, recently approved to treat platinum-resistant ovarian cancer: 2k medication dispenses + administrations
- Genetic testing: 35k+ BRCA test results
- Total abdominal hysterectomy with bilateral salpingo-oophorectomy (TAH-BSO): 35k procedures
- Patients with an ovarian cancer diagnosis with household income less than $50K: 22k patients
Truveta’s nationally representative, daily updated data includes a full demographic breakdown of the ovarian cancer population which includes:
Comorbidities: 68K patients with obesity, 22k patients with type II diabetes, 56k patients with hypertension
Race: 72.1% white, 9.7% Black, 4.2% Asian
Researchers can study all types of cancer treatment including:
- Chemotherapy
- Immunotherapy
- Radiation treatment
- Hormone therapy
- Surgery
Study the largest collection of clinical notes and images at scale
With more than 5 billion clinical notes available across conditions, Truveta eliminates the need for manual chart abstraction by using expert-led AI. Researchers can incorporate data on genetic testing results (including BRCA1, BRCA2, and HRD), disease staging, adverse events, line of therapy, patient-reported outcomes, and more.
For example, with the Truveta Language Model, physician notes can be scanned at scale for concepts such as:
- Identifying lines of therapy
- Reason for therapy switch/failure
- Positive for BRCA1 or BRCA2
The sample note below demonstrates examples of highly relevant patient information that can be extracted, structured, and made available.

As for images, Truveta Data includes over 1.1 million de-identified imaging studies specific to the ovarian cancer population, all linked to the complete EHR record. With imaging playing a crucial role in the diagnosis, staging, treatment planning and follow-up of ovarian cancer patients, this data allows for more precise identification of cancer progression patterns and treatment responses.
What’s next for ovarian cancer treatment
The FDA has approved more ovarian cancer therapies since 2014 than in the 60 prior years combined. Although there is much work to be done, these newer, targeted therapies are enhancing survival rates and providing more options for women with ovarian cancer. By providing the most comprehensive view of the patient journey, Truveta empowers researchers to accelerate the adoption of new therapies, improve clinical trials, and enhance patient care.
