Sign up for a free account to read full studies, with full transparency into methods and results, and experience the power of Truveta Studio
Authors: Brianna M. Goodwin Cartwright, MS ⊕Truveta, Inc, Bellevue, WA, Patricia J. Rodriguez, PhD MPH ⊕Truveta, Inc, Bellevue, WA, Samuel Gratzl, PhD ⊕Truveta, Inc, Bellevue, WA, Charlotte Baker, DrPH MPH CPH ⊕Truveta, Inc, Bellevue, WA, Duy Do, PhD ⊕Truveta, Inc, Bellevue, WA, Nicholas Stucky, MD PhD ⊕Truveta, Inc, Bellevue, WA
Date: August, 2024
Abstract
Background
The introduction of several lower-priced biosimilar versions of Humira in 2023 was expected to result in patients switching from bio-originator (e.g., branded) to biosimilar Humira, but reported uptake of biosimilars has been low. We explore patient characteristics and switching over time, including the potential influence of a CVS formulary change to remove bio-originator Humira.
Methods
Patients taking bio-originator Humira with at least one potential switching opportunity were identified. These were defined as patients with an outpatient office or telehealth encounter since biosimilar availability (January 31, 2023), at least two dispenses of bio-originator Humira in the 12 months prior to the encounter, and who had not yet been prescribed a biosimilar by the time of the encounter.
Among this population, we describe the number of patients with any switching evidence (prescription or dispense of biosimilar) during the time period. Demographic and health characteristics of patients switching were compared to the overall population.
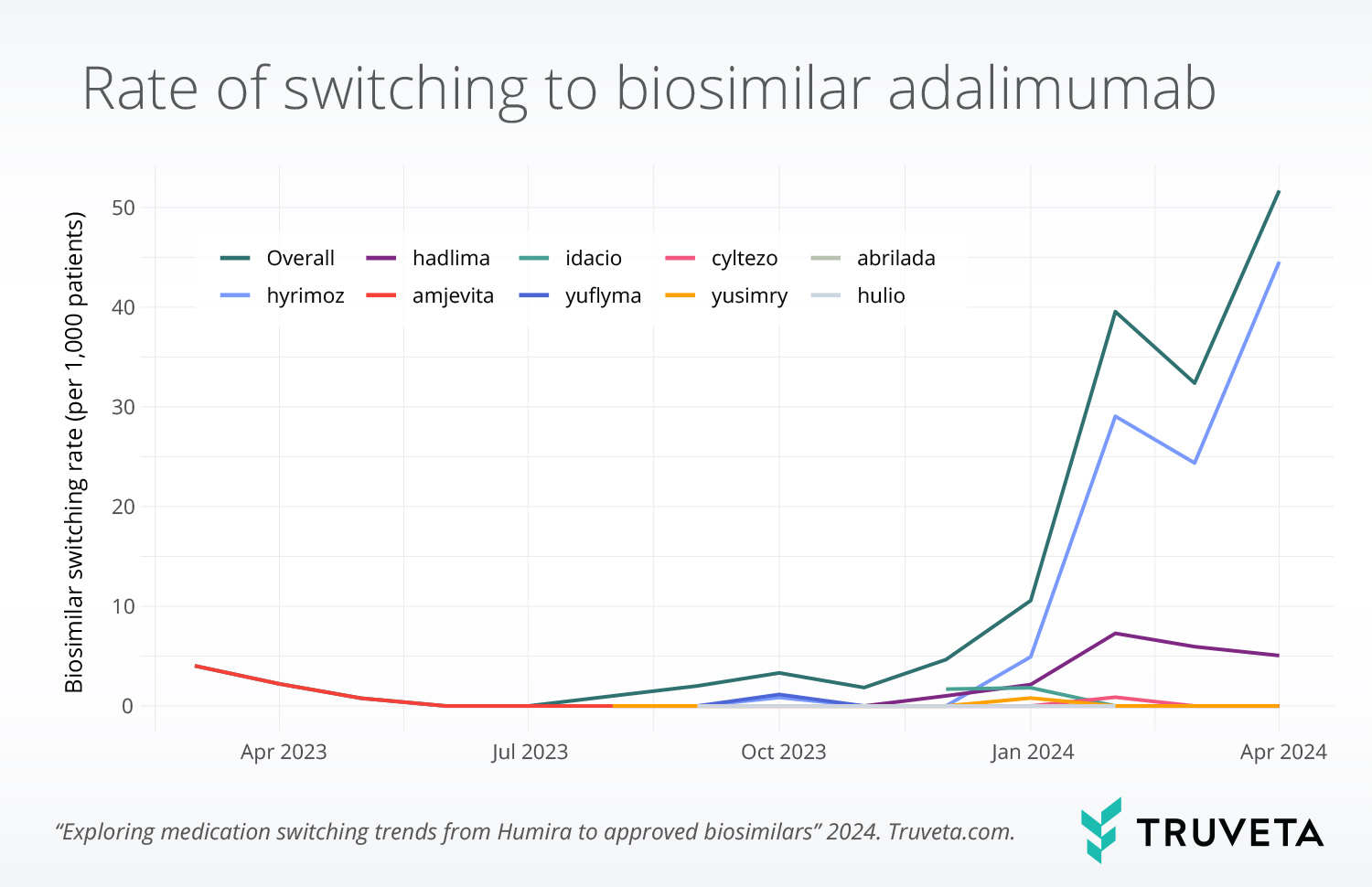
Monthly switching over time was estimated as the number of first-time switches in the month divided by the number of patients with potentially eligible encounters (as above) in the month. Rates were plotted overall and for each biosimilar. Poisson models were used to test for changes in switching overall before and after the announcement of a CVS formulary change.
Results
We identified 42,612 patients on bio-originator Humira who were potentially eligible for switching. Of these, 2,070 (4.9%) had evidence of switching to a biosimilar by April 2024. Patients who switched (cohort overall) had a median age of 50.1 (51.9) years, 59% (65%) were female, 77% (76%) were white, and 3% (4%) had an annual income of less than $25,000. Compared to the cohort overall, patients who switched had similar prevalence of psoriasis (24% in both groups; p = 0.6), and higher rates of rheumatoid arthritis (35% of those who switched vs. 32% overall; p = 0.004), Crohn’s disease (21% vs. 16%; p < 0.01), psoriatic arthritis (17% vs. 14%; p = <0.001), ankylosing spondylitis (12% vs. 8%; p = <0.01), and ulcerative colitis (12% vs. 9%; p <0.01). Conditions were not mutually exclusive.
Switching to biosimilars was minimal until January 2024, when use increased significantly (p <.001). This corresponds with the CVS announcement that bio-originator adalimumab would be removed from the formulary. Since January 2024, 77% of all switches were to Hymiroz, the biosimilar favored by CVS.
Sign up for a free account to read this full study, with full transparency into methods and results, and experience the power of Truveta Studio