Solutions
Life science
Accelerate therapy development and adoption
Generate regulatory grade evidence across the product lifecycle with the most representative, complete, and timely patient journey data.
How we can help
Safety
Fulfill post-market regulatory requirements and accelerate safety assessments with real-time data.
- Streamline post-market safety requirements, including PASS and PMR studies, reducing reliance on costly trials and registries.
- Quickly identify and assess potential safety signals using real-time data.
- Assess long-term safety across diverse patient populations with complete EHR data linked to closed claims, SDOH, and mortality data.
- Predict adverse event risks and simplify case processing with AI modeling.
Assessing the safety of novel interventions
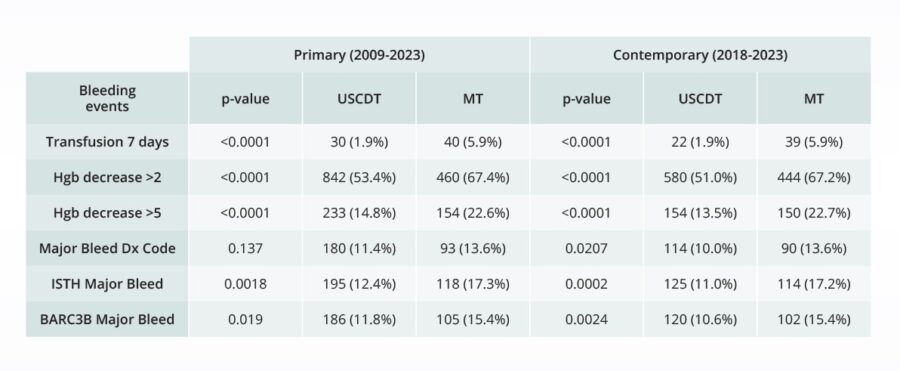 Comparative bleeding incidence 7 days post-procedure for pulmonary embolism interventions
Comparative bleeding incidence 7 days post-procedure for pulmonary embolism interventions
HEOR
Generate robust evidence on cost-effectiveness and outcomes to accelerate regulatory approval and reimbursement.
- Leverage linked EHR and closed claims, alongside AI, to evaluate real-world outcomes and cost effectiveness of drugs and devices.
- Generate evidence to support reimbursement decisions, including CMS national coverage determinations and label/indication expansion.
- Train AI models to predict treatment response, disease progression, and long-term outcomes.
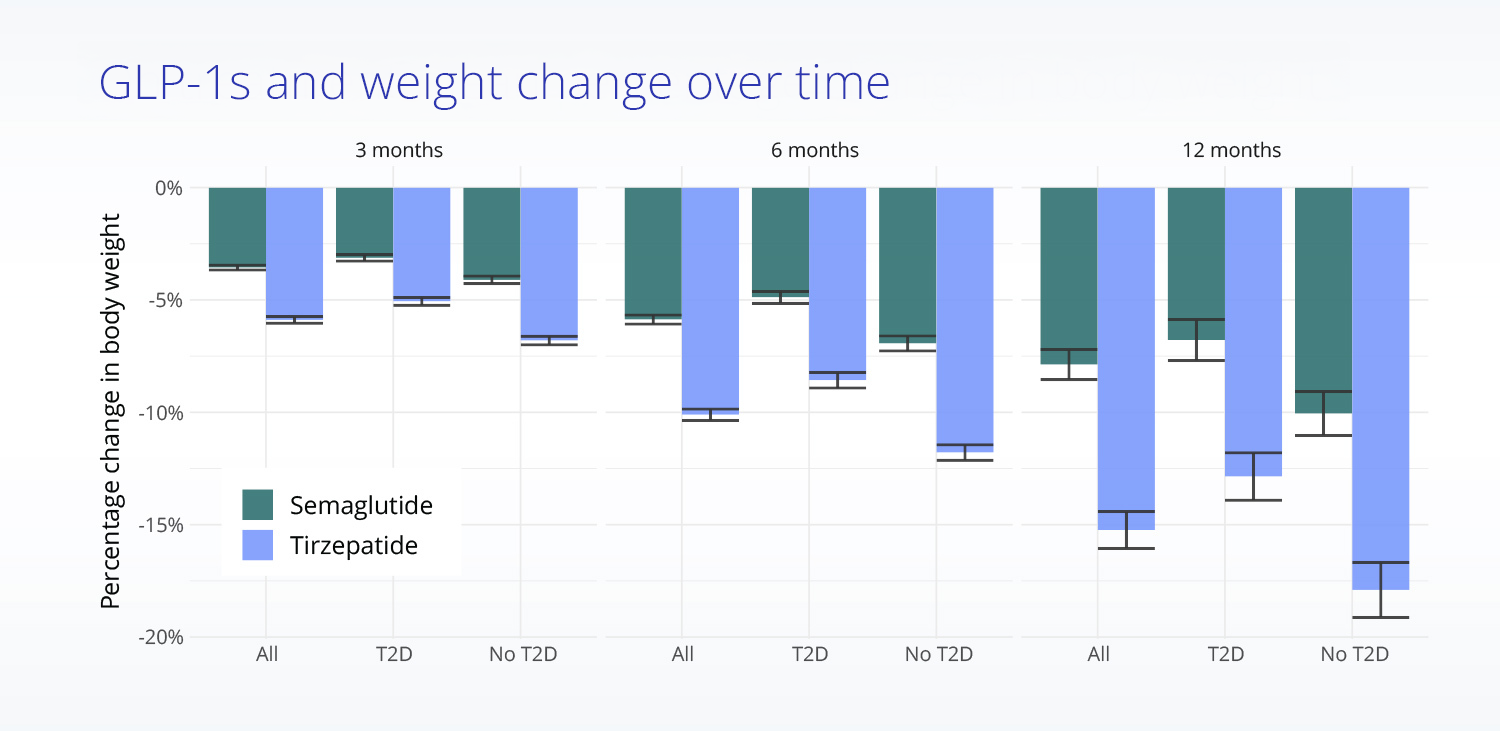
Comparing real-world treatment effectiveness
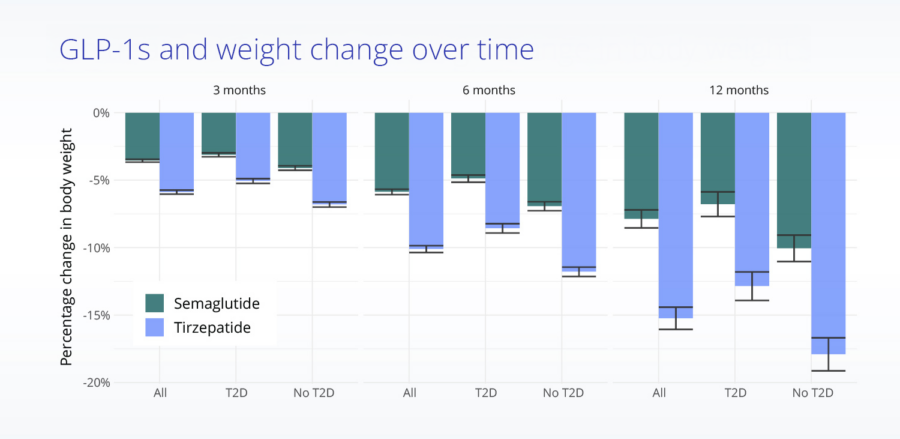
Mean body weight change from baseline in treated, propensity score matched patients.
Clinical trials
Accelerate trial design, reduce risk, and enhance trial evidence with high-quality real-world data.
- Dynamically test and refine trial cohorts by adjusting eligibility criteria and assessing real-world impact.
- Accelerate patient recruitment via direct engagement with Truveta’s health system members.
- Supplement or replace traditional control arms with longitudinal, regulatory grade data.
Testing I/E criteria to de-risk clinical programs

Sample heart failure population with inclusion/exclusion criteria applied
R&D
- Identify clinically relevant biomarkers and precision medicine opportunities with integrated genomic and phenotypic data.
- Track real-world disease progression to optimize early-stage development.
- Train AI models to accelerate target discovery and device innovation with data from representative populations.
Genetic association Manhattan plot
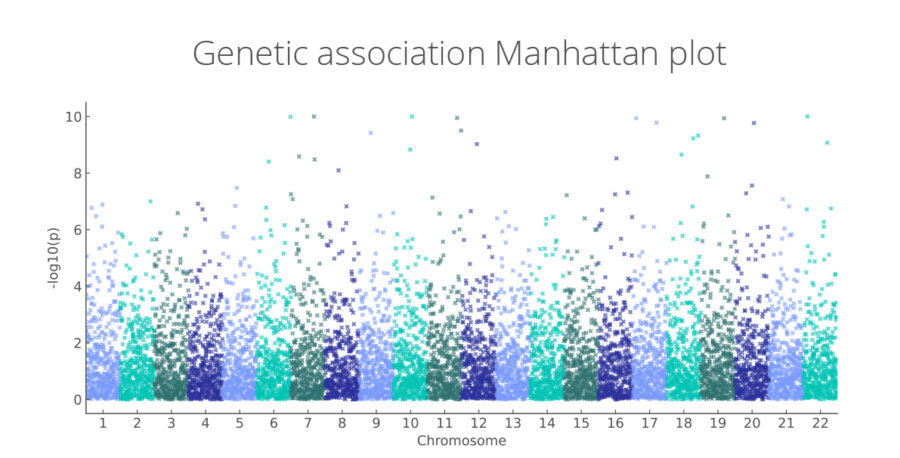
Genome-wide associations linking genetic variants to disease risk and therapeutic targets
Close care gaps
Monitor real-world treatment patterns, access barriers, and market dynamics to maximize patient reach.
- Analyze real-world treatment patterns and barriers to timely intervention to accelerate therapy adoption.
- Evaluate disparities in access by examining utilization trends across demographics, geography, and payer types.
- Develop medical education addressing trends in patient adherence, discontinuation, and switching.
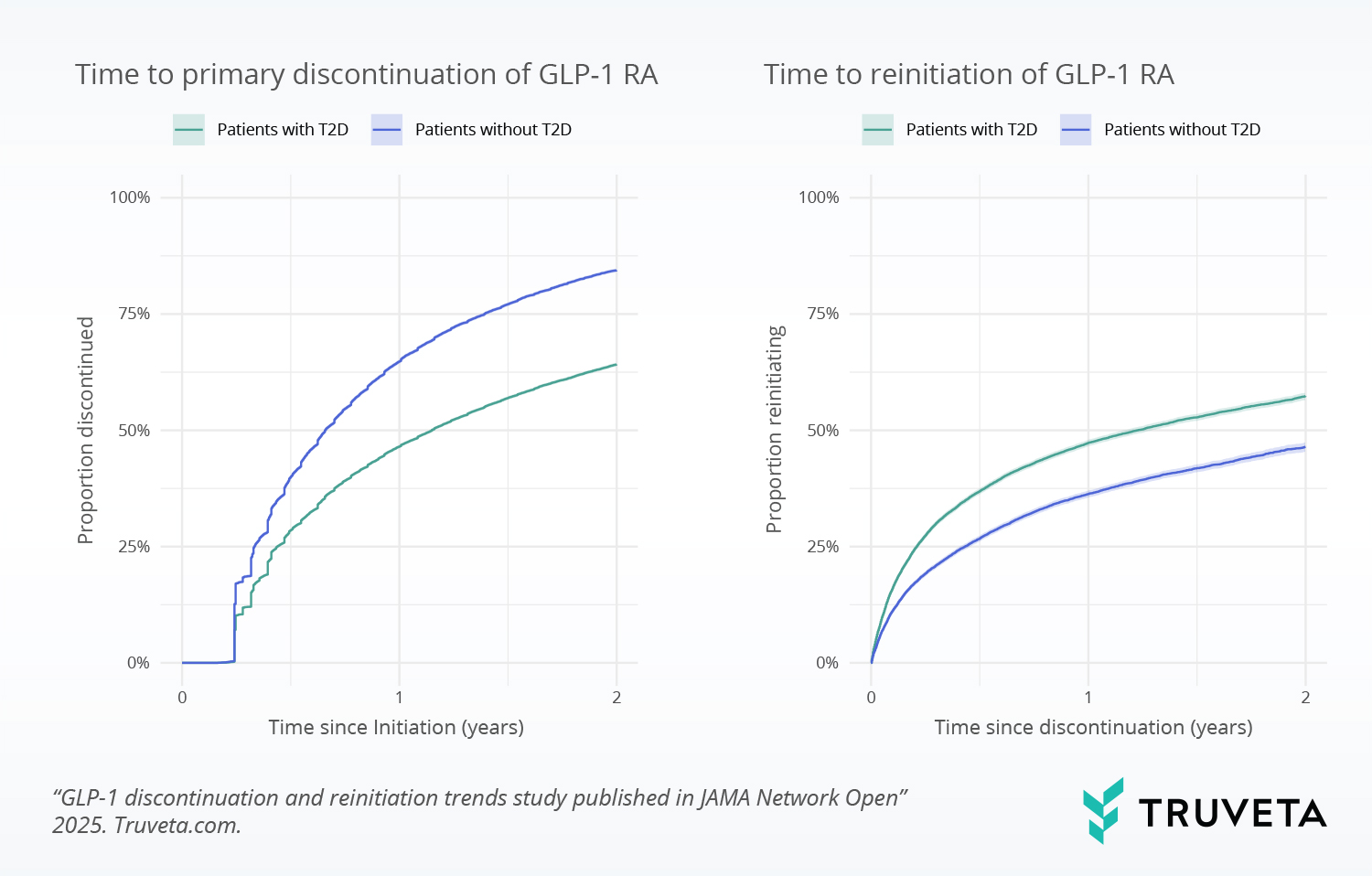
Analyzing therapy discontinuation and reinitiation trends
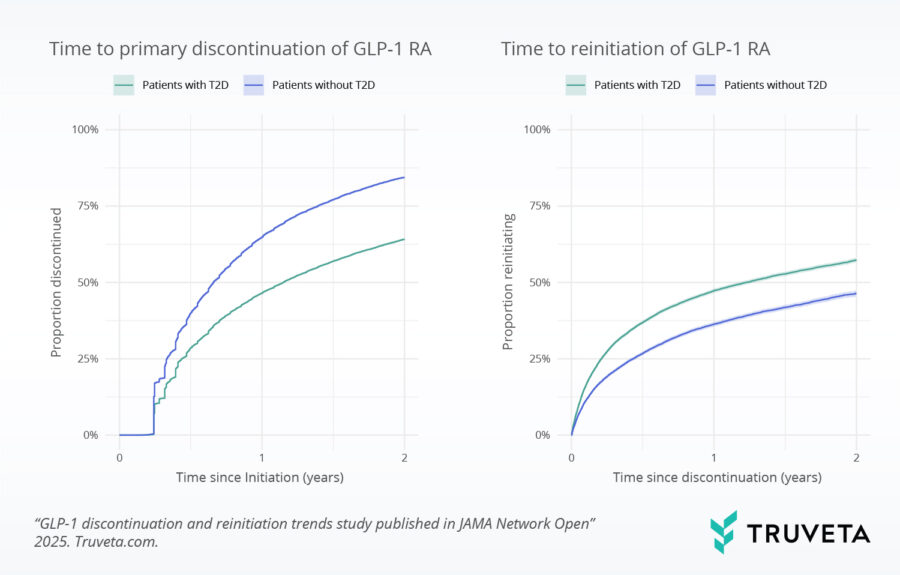
Proportion of patients discontinuing and reinitiating GLP-1 RAs within 2 years
How we can help

Safety

HEOR

Clinical trials

R&D

Close care gaps
Safety
Fulfill post-market regulatory requirements and accelerate safety assessments with real-time data.
Streamline post-market safety requirements, including PASS and PMR studies, reducing reliance on costly trials and registries.
Assessing the safety of novel interventions
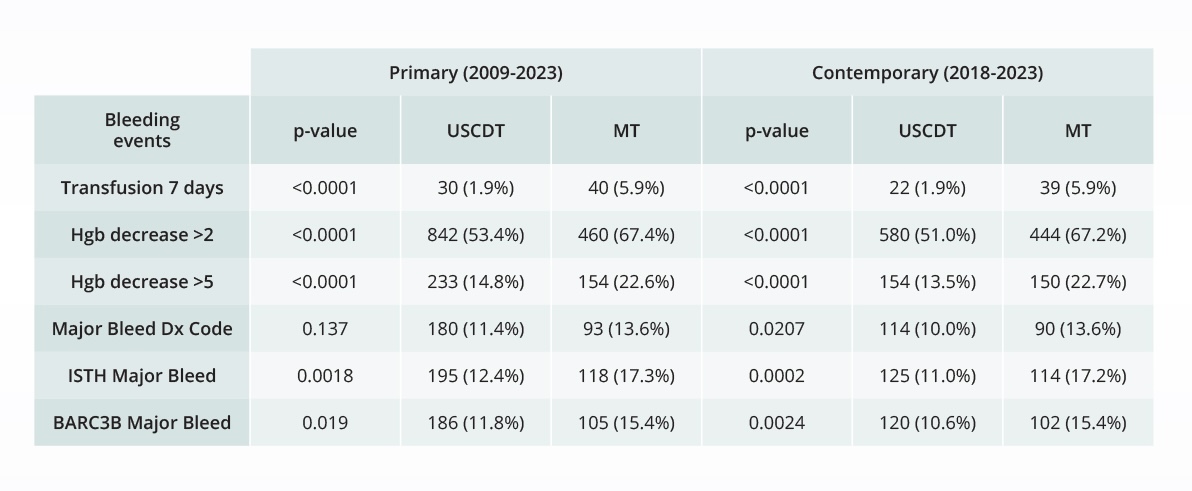
Comparative bleeding incidence 7 days post-procedure for pulmonary embolism interventions
HEOR
Generate robust evidence on cost-effectiveness and outcomes to accelerate regulatory approval and reimbursement.
Leverage linked EHR and closed claims, alongside AI, to evaluate real-world outcomes and cost effectiveness of drugs and devices.
Generate evidence to support reimbursement decisions, including CMS national coverage determinations and label/indication expansion.
Clinical trials
Accelerate trial design, reduce risk, and enhance trial evidence with high-quality real-world data.
Dynamically test and refine trial cohorts by adjusting eligibility criteria and assessing real-world impact.
Testing I/E criteria to de-risk clinical programs

Sample heart failure population with inclusion/exclusion criteria applied
R&D
Accelerate drug and device innovation with AI and linked EHR and multiomics, closed claims, and SDOH data.
![manhattan_plot_chr1_22[62] Population clustering visualization featuring two panes in Truveta Studio. The first pane explores the absence or presence of Type 2 diabetes, with distinct clusters or patterns representing different groups within the population. The second pane depicts heart failure rates, offering insights into how these rates vary or cluster within the analyzed population. The visualization provides a comprehensive overview of the relationships between Type 2 diabetes and heart failure prevalence.](/wp-content/uploads/2025/04/manhattan_plot_chr1_2262.jpg)
Close care gaps
Monitor real-world treatment patterns, access barriers, and market dynamics to maximize patient reach.
Why Truveta

Complete data

Regulatory grade, audit ready

Notes and images
With access to 7B+ notes and 100M+ images integrated with EHR data, researchers can access unprecedented depth of clinical context for patient journeys.