Solutions
Public health
Improve population health and advance patient care
Utilize real-world data, powerful analytics, and the Truveta Language Model to advance public health
How we can help
Safety
Monitor patient access and therapy responses with research-ready data, updated daily for real-time insights.
- Quickly assess emerging safety signals with real-time data
- Quantify rates of adverse events for any therapeutic
- Assess how safety signals differ by clinical or demographic groups
- Conduct studies assessing the long-term safety of different treatments
- Monitor post-approval safety and outcomes for any drug or device
- Assess safety of medications in pregnant women and their infants
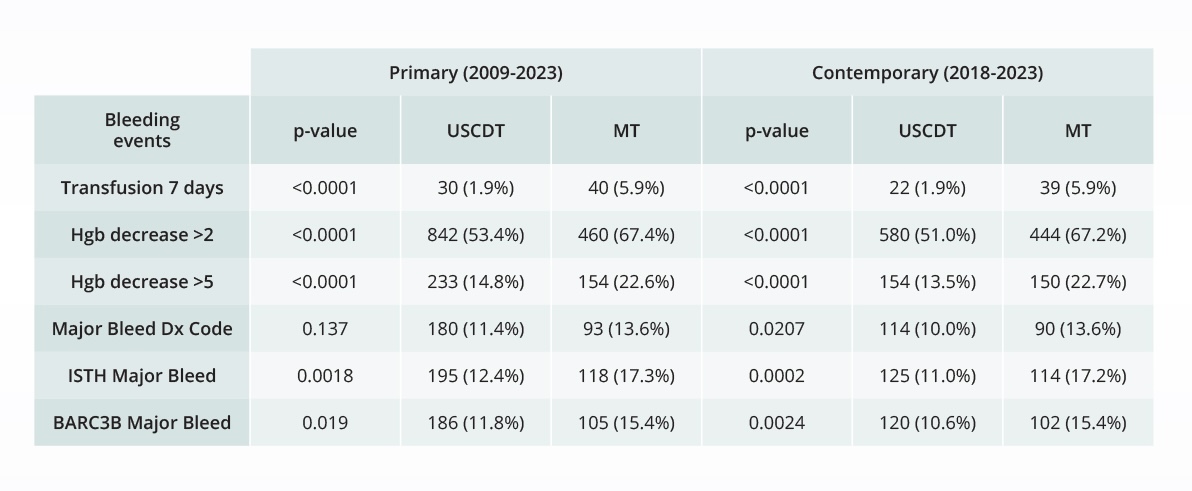
Assessing the safety of novel interventions
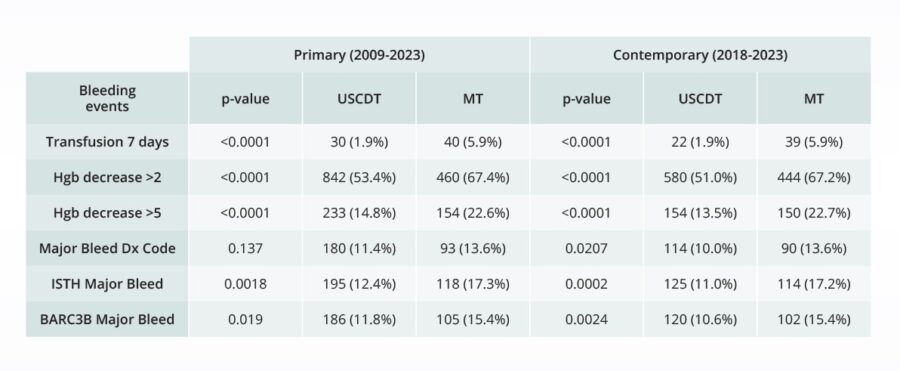 Comparative bleeding incidence 7 days post-procedure for pulmonary embolism interventions
Comparative bleeding incidence 7 days post-procedure for pulmonary embolism interventions
Comparative effectiveness
- Assess treatment efficacy with timely, patient-level outcomes and SDOH data
- Monitor performance of different therapeutics in real-world clinical environments
- Analyze how access and utilization differ between groups and across geographies
- Compare disease progression and treatment with access to longitudinal, complete data
- Understand treatment response across diverse patient populations
- Guide policy and reimbursement decisions with access to complete clinical data
Comparing effectiveness of tirzepatide (Mounjaro) and semaglutide (Ozempic) for weight loss
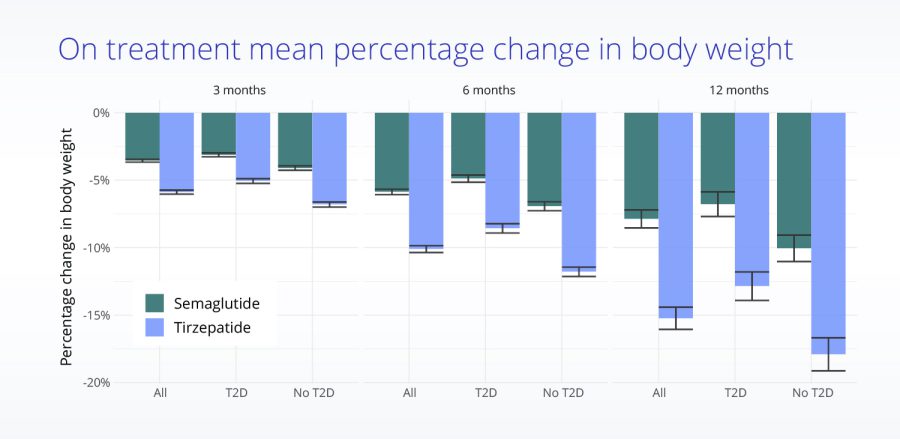 Comparing mean percentage body weight loss for patients taking semaglutide (Ozempic) and tirzepatide (Mounjaro)
Comparing mean percentage body weight loss for patients taking semaglutide (Ozempic) and tirzepatide (Mounjaro)
Clinical research
- Supplement trial insights with real-world control arms
- De-risk regulatory decisions with dynamic, real-time analytics
- Understand variations in trial site and participants
- Support and assess regulatory decisions with access to daily-updated data
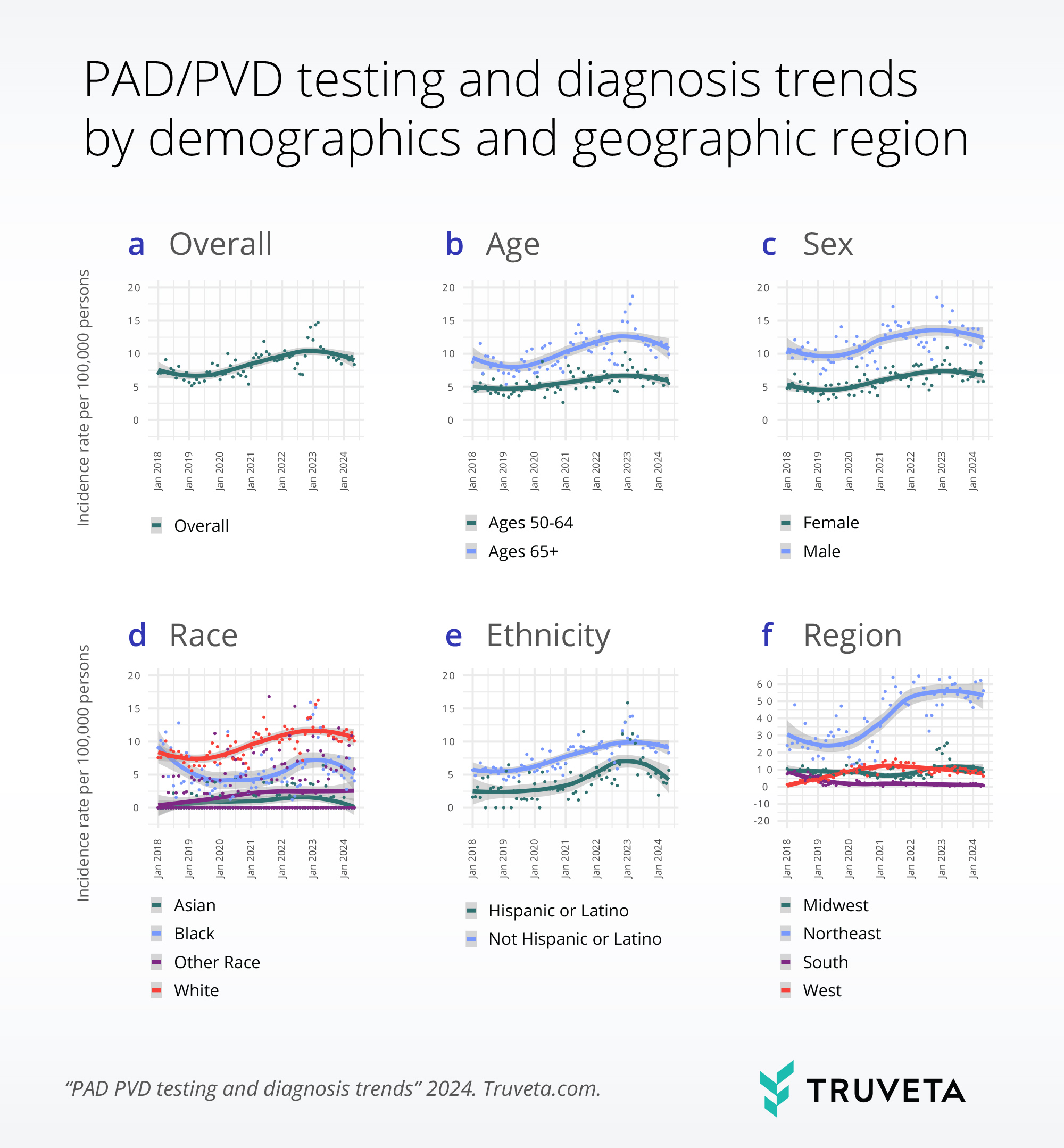
De-risking clinical trials by dynamically testing inclusion/exclusion (I/E) criteria 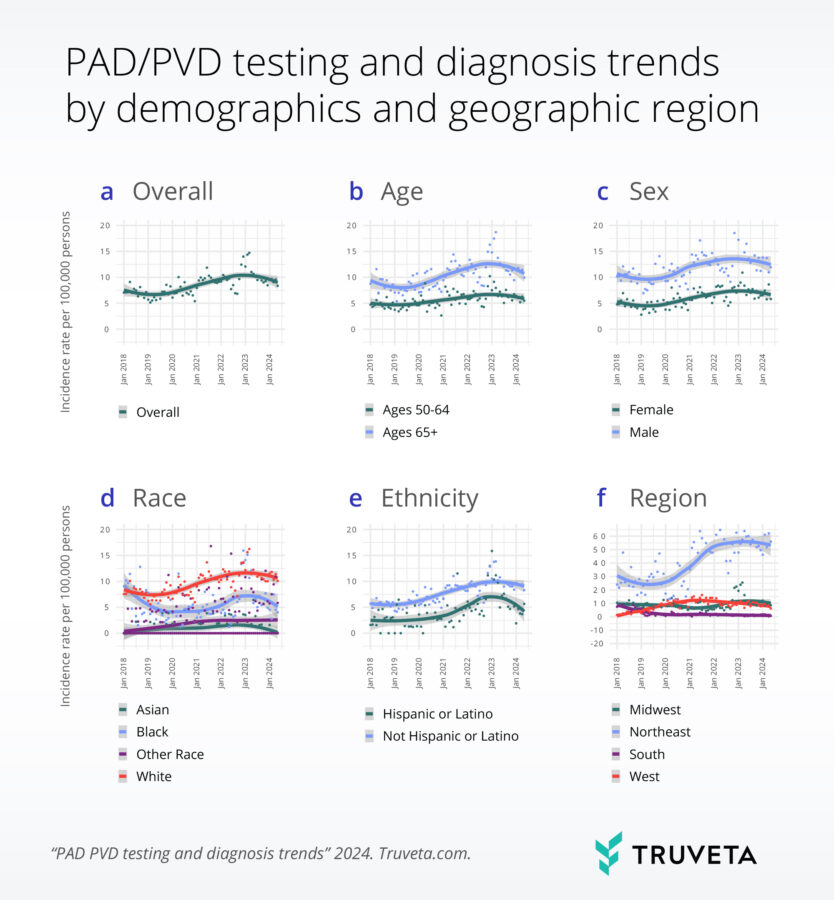 Understanding the incindence of testing and diagnosis of PVD/PAD by selected patient characteristics
Understanding the incindence of testing and diagnosis of PVD/PAD by selected patient characteristics
Population health
Monitor population health trends, access to care, and respond to emerging health threats
- Perform real-time surveillance of screening, diagnoses, and treatment patterns
- Prepare rapid response programs with real-time monitoring across demographics and geographies
- Monitor drug shortages and identify trends in drug prescriptions and fill rates
- Guide resource allocation, evaluate policy, and identify disparities in access and outcomes
- Evaluate AI model accuracy and representativeness over time with representative, real-world data
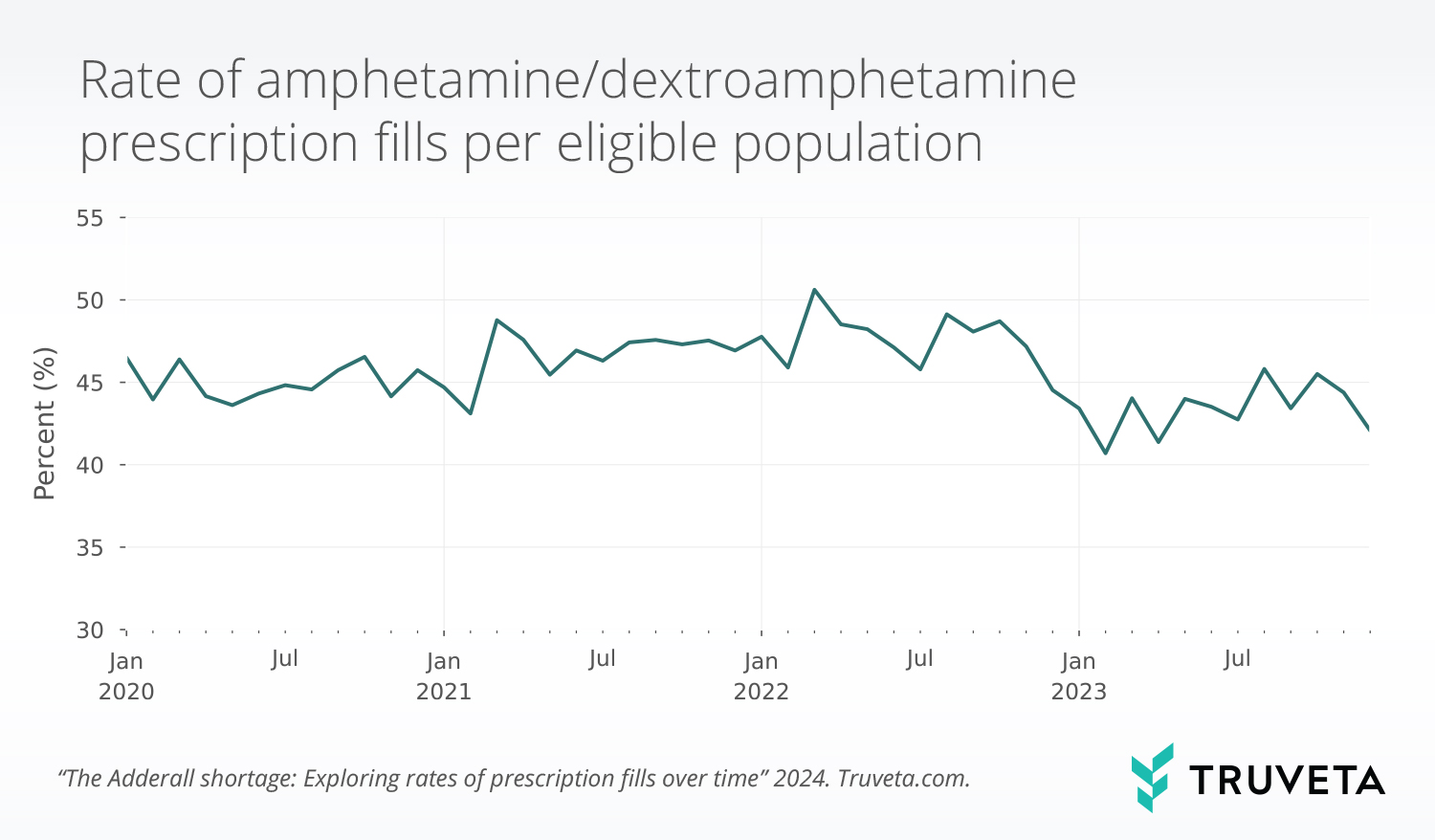
Assessing the impact of drug shortages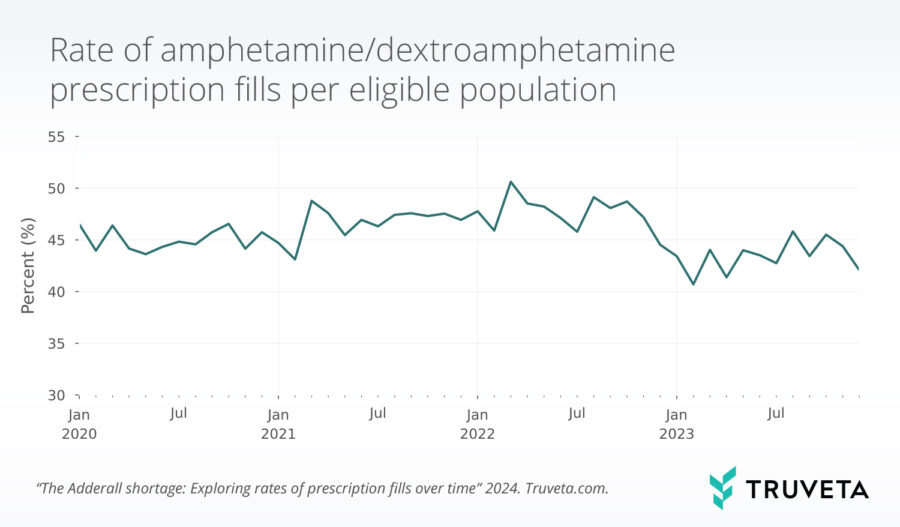 Rate of ampheatmine/dextroamphetamine prescription fills per eligible population
Rate of ampheatmine/dextroamphetamine prescription fills per eligible population
Care quality
Monitor population health trends, identify gaps in access to treatment, and inform policy.
- Assess outcomes of treatments or interventions to inform public policy
- Discover patterns or gaps in care quality across demographic groups
- Design and validate new guidelines and quality measures using granular clinical data
- Identify and analyze quality indicators to evaluate policy effectiveness
- Inform value-based care models with access to real-time data across care settings
Understanding risk of breakthrough infection and hospitalization across populations
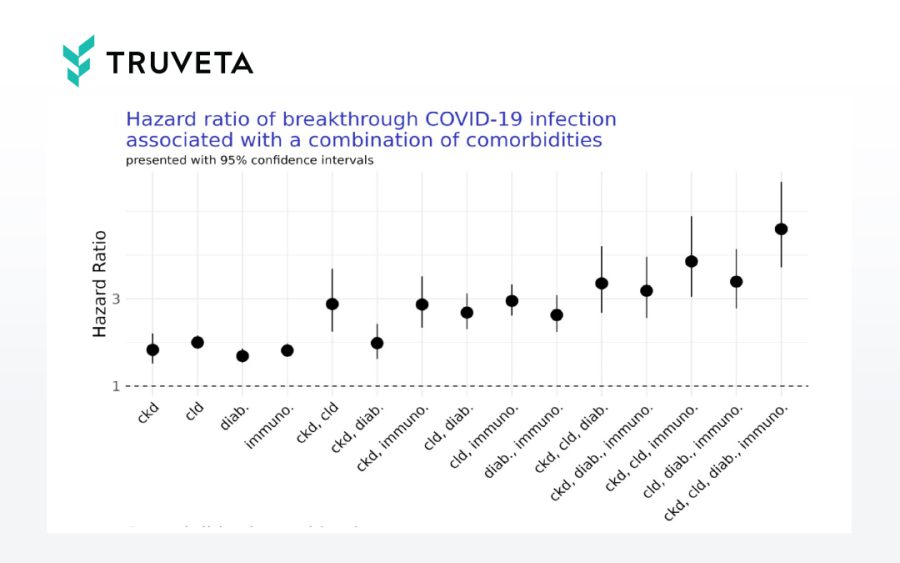 Hazard ratio of breakthrough COVID-19 infection associated with a combination of comorbidities
Hazard ratio of breakthrough COVID-19 infection associated with a combination of comorbidities
How we can help

Safety

Comparative effectiveness

Clinical research

Population health

Care quality
Safety
Monitor patient access and therapy responses with research-ready data, updated daily for real-time insights.
Comparative effectiveness
Assess the clinical and cost-effectiveness of products by leveraging real-world data from over 120 million patients to drive meaningful differentiation.
Assess treatment efficacy with timely, patient-level outcomes and SDOH data
Monitor performance of different therapeutics in real-world clinical environments
Analyze how access and utilization differ between groups and across geographies
Compare disease progression and treatment with access to longitudinal, complete data
Understand treatment response across diverse patient populations
Guide policy and reimbursement decisions with access to complete clinical data

Clinical research
Supplement regulatory decision making with data from real-world clinical settings.
Population health
Monitor population health trends, access to care, and respond to emerging health threats
Monitor drug shortages and identify trends in drug prescriptions and fill rates
Guide resource allocation, evaluate policy, and identify disparities in access and outcomes
Evaluate AI model accuracy and representativeness over time with representative, real-world data
Care quality
Monitor population health trends, identify gaps in access to treatment, and inform policy.
Design and validate new guidelines and quality measures using granular clinical data

Why Truveta

Complete EHR data
Truveta offers complete and clean EHR data linked with SDOH, mortality, and claims data, with rigorous standards for data quality.

Representative data, updated daily
Truveta Data is updated daily from health systems, representing the full diversity of the US.