Truveta Data
Regulatory-grade
Supporting regulatory submissions and audit readiness
Generate evidence with regulatory-grade EHR data
While clinical trials have been the gold standard for regulatory decision making, real-world data offers critical and timely insights into product performance and safety in real-world settings.
Truveta Data, the most complete, timely, and clean regulatory-grade EHR data for more than 120 million patients across the US, generates real-world evidence that can help accelerate therapy approval and adoption.
Truveta Data is research-ready for regulatory-grade submissions and audits
Aligned with final guidance published by the FDA, Truveta established rigorous standards of data quality and provenance and audit-ready processes, procedures, and controls to support organizations in meeting the most stringent regulatory requirements.
EHR patients and growing

closed claims patients and growing

Accurately linked across
50M+
patients and growing
Updated daily, expert de-identified, and immediately available

Robust quality management system exceeds regulatory requirements for data integrity, with continuous improvements across metrics of representativeness, completeness, cleanliness, and timeliness.

Accurate, transparent, and accountable Truveta Language Model cleans billions of daily EHR data points and concepts from clinical notes. Each concept is accompanied with detailed documentation of definitions, modeling methods, and precision and recall of extraction.

Data is protected through every stage of regulatory submissions and potential audits. Truveta has earned Type 2 SOC 2 attestation and ISO 27001, 27018, and 27701 certifications, with additional certifications underway.

All data and documentation are stored for the duration required by the FDA and are accessible by the researcher if or when an audit occurs.

Full transparency into provenance of each data point, given Truveta’s unique relationship with health system members.
Deborah Hammond
Gathering evidence in Truveta Studio
Truveta Studio supports customers at each stage of the regulatory process, including artifact creation, regulatory submission, and any regulatory audits.

Customers can classify a study, population snapshot, or notebook analysis for regulatory submission, making the study identifiable as a regulatory artifact.


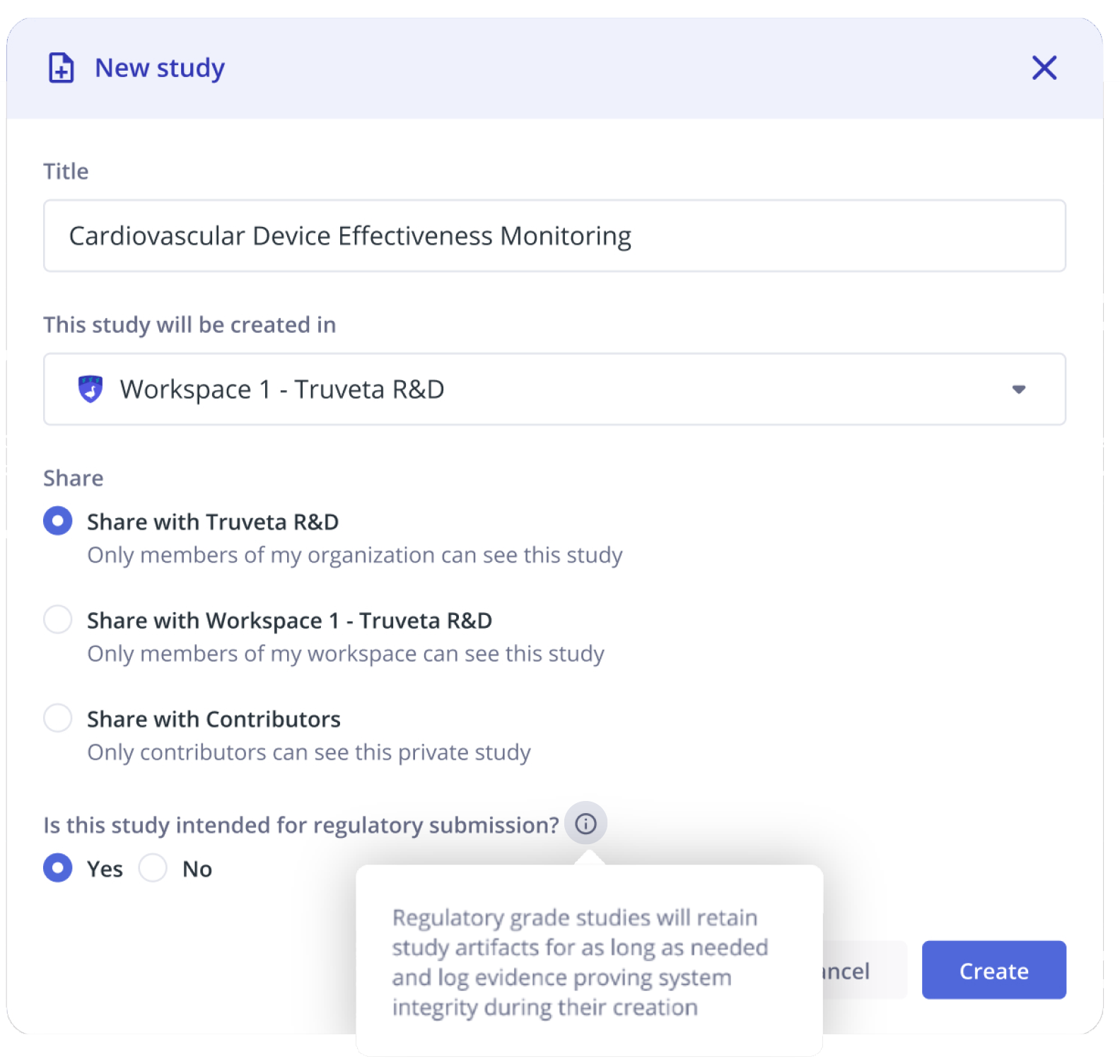
Saving a study for regulatory submission in Truveta Studio, enabling features for evidence generation, exports, and data retention policies

Saving a study for regulatory submission in Truveta Studio, enabling features for evidence generation, exports, and data retention policies

Customers can classify a study, population snapshot, or notebook analysis for regulatory submission, making the study identifiable as a regulatory artifact.

Regulatory artifacts are stored in read-only, verifiable exports, with logged evidence of system integrity accessible for audits.

All logged evidence proving the integrity of system processes is stored and readily available and can be provided easily to an auditor upon request.
Professional support services for regulatory submissions
In addition to embracing a growing partner ecosystem, Truveta offers regulatory professional services.

Extensive experience encompassing over 170 publications, nearly 11,000 citations, more than 200 conference presentations and posters, and numerous regulatory projects.

Life science organizations can use Truveta to advance safety, health economics and outcomes research (HEOR), and clinical trial solutions.

Support for post-marketing commitments/requirements (PMC/PMR) and post-authorization safety/efficacy studies (PASS/PAES).
Explore whitepapers and briefings

Data quality

Data security

Data analytics

Generating RWE for regulatory submission
Learn more about the depth of Truveta Data
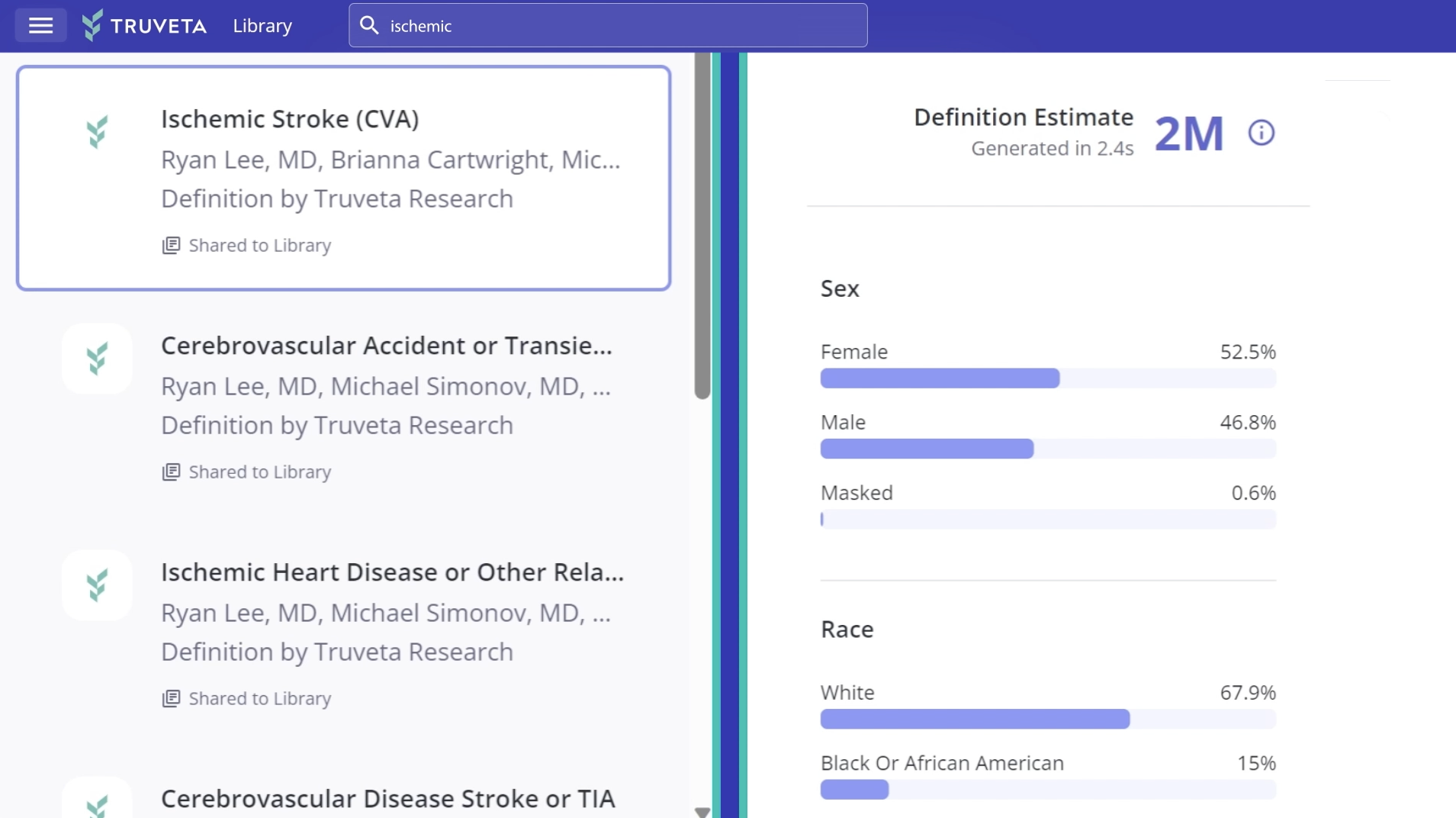
Complete and clean EHR data
Truveta offers complete, timely, and clean EHR data linked with SDOH, mortality, and claims data for more than 120M patients representing the full diversity of the US.

Data cleaned with AI
Truveta Language Model, a large-language, multi-modal AI model, transforms billions of data points with industry-leading normalization.

